Summary
This article discusses the relationship between glucagon-like peptide-1 (GLP-1) and both cardiovascular disease, as well as the central nervous system.
- Hyperglycemia/Hypoglycemia
- Obesity
- Diabetes Mellitus Diabetes & Metabolic Syndrome
Cardiovascular disease (CVD) is a leading cause of death in patients with diabetes mellitus [Yoon JS, Lee HW. Diabetes Metab J 2011]. Mansoor Husain, MD, University of Toronto, Toronto, Ontario, Canada, reported on the relationship between type 2 diabetes (T2DM) and CVD; the CV effects of glucagon-like peptide-1 (GLP-1), dipeptidyl peptidase 4 inhibitor (DPP-4i), and glucagon-like peptide-1 receptor agonist (GLP-1RA) in experimental systems; the CV effects of GLP-1, DPP-4i, and GLP-1RA in humans; and findings from meta-analyses and CV outcome trials. His main focus was on the role of novel therapeutic agents that target the incretin pathway in humans.
Incretin-based treatments have garnered much interest due to use-associated weight loss (GLP-1 agonists), minimal hypoglycemia, and the potential for positive effects on pancreatic β-cell biology and the CV system [Phillips LK, Prins JB. Ann NY Acad Sci 2011]. Although GLP-1 and GLP-1RAs have demonstrated beneficial effects on myocardium and vascular endothelium, including coronary and peripheral mouse vessels, they also have anti-inflammatory and anti-atherogenic actions, and positive effects on lipid profile and blood pressure [Yoon JS, Lee HW. Diabetes Metab J 2011].
The randomized, double-blind, placebo-controlled study To Evaluate the Effect of Liraglutide Versus Glimepiride on Haemoglobin A1C [LEAD-3 Mono; Garber A et al. Lancet 2009] showed that liraglutide is safe and effective as initial pharmacological therapy T2DM diabetes and leads to greater reductions in HbA1C (p=0.0014) as well as weight, hypoglycemia, and blood pressure than glimepiride.
Klonoff et al. [Curr Med Res Opin 2008] found that adjunctive exenatide treatment for ≥3 years in patients with T2DM led to significant improvements in triglycerides (p=0.0003), total cholesterol (p=0.0007), high-density lipoprotein cholesterol (p<0.0001), low-density lipoprotein cholesterol (p<0.0001), systolic blood pressure (p=0.0063), and diastolic blood pressure (p<0.0001).
A recent trial by Gupta et al. [Platelets 2012] found that platelets from 10 normal humans pretreated with 5 and 10 μg/mL of sitagliptin showed 25%±4% and 40%±6% inhibition of thrombin-induced platelet aggregation, respectively. Sitagliptin decreased intracellular free calcium (2.5-fold) and tyrosine phosphorylation of multiple proteins in thrombin-induced platelet activation. The drug inhibited platelet aggregation in those with T2DM as well as healthy subjects.
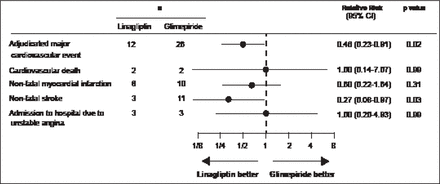
Gallwitz et al. [Lancet 2012] conducted a 2-year randomized, parallel-group, noninferiority, double-blind comparison of the efficacy and safety of the DPP-4i, linagliptin, and a commonly used sulfonylurea, glimepiride. The primary endpoint, change in HbA1C from baseline to Week 104, met the predefined noninferiority criterion of 0.35%. In addition, fewer participants had hypoglycemia (58 [7%] of 776 vs 280 [36%] of 775 patients; p<0.0001) or severe hypoglycemia (1 [<1%] vs 12 [2%]) with linagliptin compared with glimepiride. Linagliptin was also associated with significantly fewer CV events (12 vs 26 patients; RR, 0.46; 95% CI, 0.23 to 0.91; p=0.02; Figure 2)
Based on these and other data, Dr. Husain concluded that GLP-1 receptors are expressed in cardiac and vascular tissues; GLP-1 exerts cardioprotective and vasodilatory effects in isolated tissues; absence or inhibition of DPP-4 improves survival in animal models of myocardial infarction (MI); GLP-1RA reduces infarct size and improves survival in animal models of MI; and GLP-1, DPP-4i, and GLP-1RA exert effects on heart, vessel, blood pressure, and risk factors in humans. He reported that clinical trials are underway to evaluate long-term CV outcomes in diabetes.
GLP-1 and the Central Nervous System
Obesity and T2DM are 2 prevalent chronic diseases that have become major public health concerns in industrialized countries; they affect at least 16 million people in the United States alone [Siegal K, Narayan KMV. Global Health 2008]. Darleen Sandoval, PhD, University of Cincinnati, Cincinnati, Ohio, USA, presented data on the relationship between GLP-1, the central nervous system (CNS), and body mass.
Precise control of energy intake, storage, and expenditure is indispensable in keeping body weight and blood glucose concentrations within physiological ranges. Communication of the body's nutritional state by feedback signaling of peripheral organs to the CNS and the appropriate behavioral and metabolic responses initiated by the brain are pivotal processes in maintaining energy homeostasis [Jordan SD et al. Cell Mol Life Sci 2010].
Barrera et al. [J Neurosci 2011] report that the regulation of energy balance requires bidirectional communication between peripheral tissues and the CNS, an essential component of which is the gut-brain axis. Of the hormonal, neural, and nutritional signals transmitted during a meal, the preproglucagon-derived peptide GLP-1 lies at both ends of the gut-brain axis.
GLP-1 is a physiologic regulator of numerous processes, including glucose homeostasis and food intake [Baggio LL, Drucker DJ. Gastroenterology 2007; Williams DL et al. Endocrinology 2009]. Recent evidence suggests that GLP-1, which is released from intestinal L-cells after meals and is also produced in the nucleus of the solitary tract, acts as a short-term satiation signal, limiting meal size and prolonging intermeal intervals. Numerous studies also support a role for CNS GLP-1 in long-term energy balance regulation [Barerra JG et al. J Neurosci 2011].
GLP-1Rs are expressed in the periphery and in several brain areas that are implicated in the control of eating. A study by Vahl et al. [Endocrinology 2007] demonstrated that GLP-1Rs are also present in the intestine and on nerve terminals in the hepatic portal bed, indicating that peripheral GLP-1 can act in 2 different sites to inhibit eating [Punjabi M et al. Physiol Behav 2011]. Both central and peripheral administration of GLP-1 reduce food intake, with central administration of GLP-1 causing a dose-dependent reduction [Barerra JG et al. J Neurosci 2011]. Exogenous activation of CNS GLP-1Rs also reduces food consumption [Hayes MR et al. Endocrinology 2009].
Gupta [Indian J Endocrinol Metab 2012] reports that the incretins represent a large family of molecules referred to as the “glucagon superfamily of peptide hormones.” GLP-1 mediates its effects via the GLP-1R, which has a wide tissue distribution that includes the CNS (neocortex, cerebellum, hypothalamus, hippocampus, and brainstem nucleus tractus solitarius). Evidence indicates that therapies that augment the incretin system have beneficial pleiotropic effects.
Relative Risk of CV Events in Treated Patients.
Reproduced from Gallwitz B et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet 2012; 380(9840):475–483. With permission from Elsevier.
- © 2012 MD Conference Express®