Summary
The once-weekly formulation of exenatide, a glucagon-like peptide-1 receptor agonist, is associated with clinically sustained improvement in glycemic control, continued improvements in cardiometabolic risk factors and weight loss after 4 years of treatment in type 2 diabetes mellitus (T2DM) patients. This article discusses results of the open-label extension of the Effects of Exenatide Long-Acting Release on Glucose Control and Safety in Subjects with Type 2 Diabetes Mellitus [DURATION-1; NCT00308139] clinical trial.
- Diabetes Mellitus
- Cardiometabolic Disorder
- Diabetes & Endocrinology Clinical Trials
- Obesity Diabetes & Metabolic Syndrome
The once-weekly formulation of exenatide, a glucagon-like peptide-1 receptor agonist, is associated with clinically sustained improvement in glycemic control, continued improvements in cardiometabolic risk factors and weight loss after 4 years of treatment in type 2 diabetes mellitus (T2DM) patients. Results of the open-label extension of the Effects of Exenatide Long-Acting Release on Glucose Control and Safety in Subjects with Type 2 Diabetes Mellitus [DURATION-1; NCT00308139] clinical trial were presented by Leigh MacConell, PhD, Amylin Pharmaceuticals, San Diego, California, USA.
In the DURATION-1 trial, treatment with exenatide once weekly (EQW) for 30 weeks significantly reduced HbA1C compared with twice-daily exenatide in patients with T2DM [Drucker DJ et al. Lancet 2008]. The study included patients with T2DM and HbA1C 7.1% to 11.0%, who were treated with diet and exercise and/or a stable dose of metformin, sulfonylurea, thiazolidinedione, or a combination of these therapies. Patients were randomized to receive EQW (2 mg) or exenatide BID (5 μg for 4 weeks, then 10 μg for 26 weeks). The primary endpoint (HbA1C) was assessed at 30 weeks, after which all patients received EQW (2 mg).
The objectives of the current analysis were to examine the long-term safety and efficacy of EQW over 4 years in patients with T2DM. Study endpoints included change from baseline to Year 4 in HbA1C, fasting plasma glucose (FPG), weight, blood pressure (BP), and lipid profile. Sixty percent (n=176) of the intention-to-treat (ITT) population completed 4 years of EQW treatment. The efficacy results are based on the 176 study completers. Safety data are based on the ITT population (n=295). Baseline characteristics were consistent between the ITT and completer populations: mean age 56 years, 54% men, mostly white, mean HbA1C 8.2%, mean FPG 9.2 mmol/L, and duration of diabetes 7 years. Most study participants received metformin (33%) or combination therapies (39%).
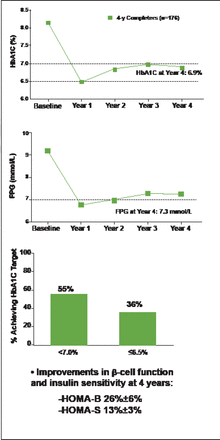
At 4 years, mean HbA1C (± standard error) was 6.9% (±0.1%), with 55% of patients achieving HbA1C <7.0% and 36% achieving HbA1C ≤6.5% (Figure 1). Clinically significant (p<0.05) improvements in FPG (−1.9 mmol) and weight (−2.5 kg) were observed. Improvements in β-cell function and insulin sensitivity were indicated by increases in the Homeostasis Model Assessment for β-cell function (HOMA-B; 26%±6%) and for insulin sensitivity (HOMA-S; 13%±3%). These changes from baseline were observed at Year 1 and maintained thereafter.
Once-Weekly Exenatide Associated with Improved HbA1C and Fasting Plasma Glucose Through 4 Years.
FPG=fasting plasma glucose; HOMA-B=Homeostasis Model Assessment B-cell function; HOMA-S=Homeostasis Model Assessment, insulin sensitivity; SE=standard error.
Reproduced with permission from Amylin Pharmaceuticals.
Improvements (baseline to 4 years) were also observed for cardiovascular risk markers: systolic BP (−1.6 mm Hg; −8.7 mm Hg in patients with abnormal baseline systolic BP), diastolic BP (−2.7 mm Hg), total cholesterol (−0.30 mmol/L), low-density lipoprotein cholesterol (−0.20 mmol/L), high-density lipoprotein cholesterol (+0.05 mmol/L), and triglycerides (−13%). Maximum response was seen at Year 2 and maintained thereafter. Seventy-one percent of patients lost weight (−2.5 kg mean weight loss at Year 4).
Nausea and injection-site pruritus—the most common adverse events (AEs)—decreased in incidence with ongoing therapy, as did vomiting and diarrhea. The annual event rate for nausea and injection-site pruritus was 15/100 years and 6/100 years patient exposure over the 4-year study duration. Cardiac and renal/urinary disorders occurred at event rates of 5 and 6 per 100 years patient exposure, respectively. Twenty percent of EQW patients experienced serious AEs (no identifiable pattern of types of events) and 3 patients died (none due to treatment). Withdrawal rates over the 4-year duration due to AEs were low (8%); gastrointestinal AEs led to withdrawal in few (2%) patients. There was no major hypoglycemia. Minor hypoglycemia increased minimally after 1 year of exenatide therapy. There were few minor hypoglycemia events in patients not using concomitant sulfonylurea.
Long-term exenatide treatment was associated with significant, sustained improvement in glycemic control and improvements in cardiometabolic measures, with no unexpected safety findings.
- © 2012 MD Conference Express®