Summary
Procedures using intravascular iodinated contrast media are being widely applied for both diagnostic and therapeutic purposes but represent one of the main causes of contrast-induced nephropathy (CIN) and hospital-acquired renal failure. In selected subsets of patients with major risk factors (eg, advanced chronic kidney disease, diabetes, or impending percutaneous coronary interventions), CIN risk can run as high as 50% [Marenzi G et al. Intern Emerg Med 2012]. This article presents findings on the Prevention of Contrast Renal Injury with Different Hydration Strategies [POSEIDON; NCT01218828] trial.
- Renal Disease
- Interventional Techniques & Devices
- Cardiology Clinical Trials
Procedures using intravascular iodinated contrast media are being widely applied for both diagnostic and therapeutic purposes but represent one of the main causes of contrast-induced nephropathy (CIN) and hospital-acquired renal failure. In selected subsets of patients with major risk factors (eg, advanced chronic kidney disease, diabetes, or impending percutaneous coronary interventions [PCIs]), CIN risk can run as high as 50% [Marenzi G et al. Intern Emerg Med 2012]. Somjot S. Brar, MD, MPH, Kaiser Permanente, Los Angeles, California, USA, presented findings on the Prevention of Contrast Renal Injury with Different Hydration Strategies [POSEIDON; NCT01218828] trial.
Several studies have shown that CIN is associated with increased morbidity and mortality, extended length of hospital stay, and increased costs [Gallagher S, Knight C. BMJ 2011]. CIN has no effective treatment [Marenzi G. et al. Intern Emerg Med 2012]. The hallmark of therapy is prevention, yet preventive strategies remain limited, said Dr. Brar.
The Phase 3, randomized POSEIDON trial compared standard intravenous (IV) hydration (0.9% saline) with left ventricular end diastolic pressure (LVEDP)-based hydration therapy. Questions surrounding standard IV hydration therapy include its rate and duration, and whether it can be optimized to the patient's needs. The trial's hypothesis was that LVEDP-guided hydration would reduce the incidence of CIN. LVEDP is an intravascular, hemodynamic parameter routinely measured in the cardiac catheterization laboratory, representing a patient's preload or volume status.
The single-blinded POSEIDON trial was carried out between November 2010 and July 2012 in patients undergoing angiography or PCI (inpatient and outpatient) at a high-volume tertiary care center. Inclusion criteria included estimated glomerular filtration rate <60 mL/min/1.73 m2 (by Modification of Diet in Renal Disease equation) and at least one of the following: diabetes mellitus, age >75 years, hypertension (>140/90 mm Hg or treatment), or history of congestive heart failure. The primary endpoint was a 25% or 0.5 mg/dL increase in serum creatinine on two measurements between Days 1 and 4.
In the trial, 396 patients were randomized (1:1) to either LVEDP-guided hydration (n=196) or standard hydration (n=200). Prior to the procedure, all subjects received 0.9% saline IV at a rate of 3 mL/kg for 1 hour. Standard hydration patients then received 1.5 mL/kg/hr for 4 hours post-procedure. Those with LVEDP hydration received 5, 3, or 1.5 mL/kg/hr for 4 hours based on LVEDP of <13 mm Hg, 13 to 18 mm Hg, or >18 mm Hg, respectively.
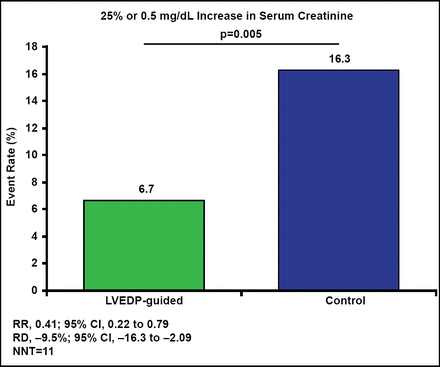
The LVEDP-guided approach significantly reduced the primary endpoint by 59% compared with conventional hydration (RR, 0.41; 95% CI, 0.22 to 0.79; p=0.005). Treating 11 patients with an LVEDP-guided hydration approach would prevent 1 case of contrast nephropathy (Figure 1).
POSEIDON Primary Endpoint.
LVEDP=left ventricular end diastolic pressure; NNT=number needed to treat.
Reproduced with permission from S Brar, MD.
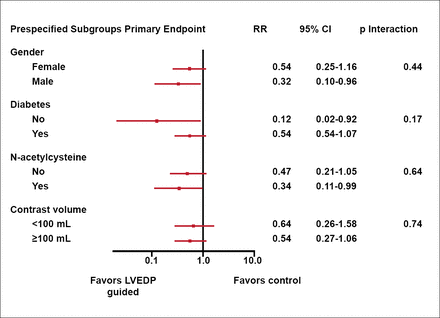
Dr. Brar pointed out that this was the first trial to test the hypothesis of an LVEDP-guided hydration strategy for prevention of CIN. In subgroup analyses, the treatment effect was also consistently in favor of LVEDP-guided hydration (Figure 2).
Outcomes of Subgroup Analyses.
LVEDP=left ventricular end diastolic pressure.
Reproduced with permission from S. Brar, MD.
- © 2012 MD Conference Express®