Summary
This article discusses the Cardiorenal Rescue Study in Acute Decompensated Heart Failure [CARRESS-HF; NCT00608491] trial that was simultaneously published in the New England Journal of Medicine [Bart BA et al. 2012].
- Heart Failure
- Cardiology Clinical Trials
- Renal Disease
Bradley A. Bart, MD, Hennepin County Medical Center, Minneapolis, Minnesota, USA, presented the Cardiorenal Rescue Study in Acute Decompensated Heart Failure [CARRESS-HF; NCT00608491] trial that was simultaneously published in the New England Journal of Medicine [Bart BA et al. 2012].
Acute cardiorenal syndrome (Type 1), defined as worsening renal function in patients with acute decompensated heart failure (ADHF) [Ronco C et al. J Am Coll Cardiol 2012], occurs in 25% to 33% of patients with ADHF and is associated with poor outcomes [Ronco C et al. J Am Coll Cardiol 2012; Metra M et al. Circ Heart Fail 2012].
CARRESS-HF was a multicenter, prospective, randomized controlled trial designed to test whether ultrafiltration was superior to stepped pharmacologic therapy for the treatment of patients with ADHF.
Patients hospitalized with ADHF and worsened renal function (defined as an increase in the serum creatinine level of at least 0.3 mg/dL) 12 weeks before or 10 days after the index admission for heart failure (HF) were eligible for inclusion. Additional inclusion criteria included at least 2 of the following conditions at the time of randomization: at least 2+ peripheral edema, jugular venous pressure greater than 10 cm of water, or pulmonary edema or pleural effusion on chest radiography.
In total, 188 patients were randomized to a strategy of stepped pharmacologic therapy (n=94) or ultrafiltration (n=94). The primary endpoint was a composite of change from baseline in serum creatinine level and body weight at 96 hours. Clinical outcomes were assessed at 60 days.
Results showed that ultrafiltration was inferior to pharmacologic therapy with respect to the primary endpoint of changes in serum creatinine and body weight at 96 hours (p=0.003); this was due primarily to an increase in creatinine levels in the ultrafiltration group.
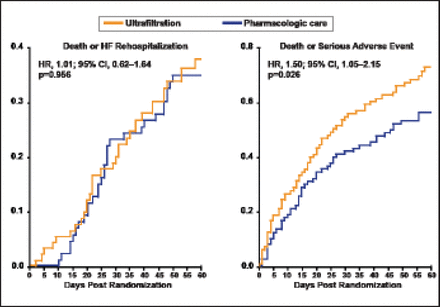
The mean change in the creatinine level at 96 days was −0.04±0.53 mg/dL in the pharmacologic therapy group compared with +0.23±0.70 mg/dL in the ultrafiltration group (p=0.003). There was no significant difference in weight loss between patients in the pharmacologic therapy group and those in the ultrafiltration group 96 hours after enrollment (a loss of 5.5±5.1 kg and 5.7±3.9 kg, respectively; p=0.58). At 60 days, there was no difference in death (17% vs 14%; p=0.55) or HF hospitalization (26% vs 26%; p=0.97), but serious adverse events (AEs) were more frequent with ultrafiltration (p=0.03) and there was a significant increase in the rate of death or serious AE with ultrafiltration compared with pharmacologic therapy (HR, 1.50; 95% CI, 1.05 to 2.15; p=0.026; Figure 1).
60-Day Outcomes Post-Randomization.
Reproduced with permission from BA Bart, MD.
Dr. Bart concluded that, compared with pharmacologic therapy, ultrafiltration as administered in this study was associated with deterioration in renal function and worse clinical outcomes, and should not be used routinely in clinical practice. Whether or not ultrafiltration could be useful using slower rates of volume removal or as guided by hemodynamics is unknown. He said, “Treatment of these patients with ADHF, worsened renal function, and persistent congestion remains a challenging clinical problem in need of better therapy.”
- © 2012 MD Conference Express®