Summary
Basic research has suggested that some individual vitamin and mineral components of multivitamins might reduce the risk of cardiovascular disease. However, no large-scale, long-term randomized trials have tested the effect of multivitamins. This article presents results from the Physicians' Health Study II (PHS II) on the long-term risks and benefits of multivitamin use in male physicians [Sesso HD et al. JAMA 2012].
- Cardiology Clinical Trials
Basic research has suggested that some individual vitamin and mineral components of multivitamins might reduce the risk of cardiovascular disease (CVD). However, no large-scale, long-term randomized trials have tested the effect of multivitamins. Howard D. Sesso, ScD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA, presented results from the Physicians' Health Study II (PHS II) on the long-term risks and benefits of multivitamin use in male physicians [Sesso HD et al. JAMA 2012].
PHS II was a randomized, double-blind, placebo-controlled, 2×2×2×2 factorial trial testing multivitamin, vitamin E, vitamin C, and beta-carotene. It was conducted by mail in 14,641 male physicians aged ≥50 years. A total of 7641 PHS I participants and 7000 new physicians were randomized to take an active multivitamin or its placebo, as well as for the other vitamin arms. For the multivitamin component, the primary cardiovascular (CV) endpoint was major CV events (nonfatal myocardial infarction [MI], nonfatal stroke, and CV death). The secondary endpoints were total and fatal MI, total and fatal stroke, ischemic and hemorrhagic stroke, CVD mortality, and total mortality. The participants were followed for a mean of 11.2 years, resulting in 164,000 person-years of follow-up.
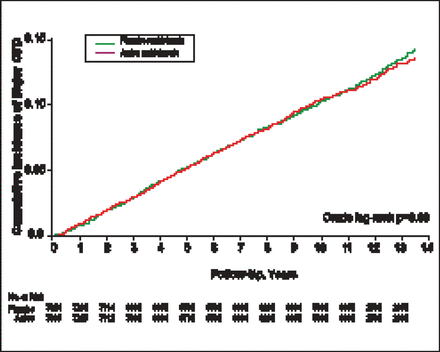
Multivitamin compliance was 77% at 4 years, 72% at 8 years, and 67% at study end. Baseline characteristics were well balanced between the multivitamin and placebo groups. The cumulative incidence of major CV events at study end was not significantly different between the 2 groups (HR, 1.01; 95% CI, 0.91 to 1.10; crude log-rank p=0.69; Figure 1). Similarly, no significant differences were seen in the incidences of secondary endpoints (Table 1). There was a borderline significant reduction in MI death (27% vs 43%; HR, 0.61; 95% CI, 0.38 to 0.995; p= 0.048) that may have due to chance, given its small case counts.
Association Between Randomized Multivitamin Assignment and Risk of Major CV Events and Mortality.a
Major CV Events: Active Versus Placebo Multivitamins During 11.2 Years of Follow-Up.
Copyright © 2001 American Medical Association. All rights reserved.
Notably, the total number of cancers—the other primary endpoint of the multivitamin component of the trial—was modestly but significantly reduced, with 1290 in the multivitamin group versus 1379 in the placebo group (HR, 0.92; 95% CI, 0.86 to 0.998; p=0.04). The total number of incident cancers among participants with a baseline history of cancer was also significantly lower in the multivitamin group (95) versus the placebo group (126; HR, 0.73; 95% CI, 0.56 to 0.96; p=0.02) but was not significantly lower among participants without a baseline history of cancer (1195 vs 1253; HR, 0.94; 95% CI, 0.87 to 1.02; p=0.15).
The results of the PHS II trial demonstrated no effect of long-term multivitamin use on CVD in men. The main reason to take a daily multivitamin is still for the prevention of vitamin and mineral deficiency, along with the potential reductions on total cancer. The investigators will provide additional results on the effects of multivitamins on the secondary endpoints of eye disease and cognitive function, and other important analyses of CV and cancer outcomes, along with extended follow-up of this trial cohort.
- © 2012 MD Conference Express®