Summary
Investigators from the Renal Insufficiency Following Contrast Media Administration Trial II [REMEDIAL II; NCT01098032] report that the RenalGuard™ automated hydration matching system is safe and effective in preventing contrast-induced acute kidney injury in high- and very-high-risk patients with chronic kidney disease compared with the optimal strategy of sodium bicarbonate infusion plus N-acetylcysteine.
- Renal Insufficiency
- Renal Disease Clinical Trials
Investigators from the Renal Insufficiency Following Contrast Media Administration Trial II (REMEDIAL II; NCT01098032) report that the RenalGuard™ automated hydration matching system is safe and effective in preventing contrast-induced acute kidney injury (CI-AKI) in high- and very-high-risk patients with chronic kidney disease (CKD) compared with the optimal strategy of sodium bicarbonate infusion plus N-acetylcysteine (NAC). Carlo Brigouri, MD, PhD, Clinica Mediterranea, Naples, Italy, presented the findings.
Contrast-induced acute kidney injury is strongly associated with unfavorable early and late clinical outcomes in patients, which may be mitigated by maintaining a high urine flow. Prior strategies focused on forced diuretic regimens (typically with high-dose furosemide), which may be harmful due to a resulting negative fluid balance. The primary hypothesis of REMEDIAL II was that achieving a precise real-time high urine output and matched fluid balance using the RenalGuard™ hydration system would be noninferior to a control hydration strategy of prophylactic sodium bicarbonate plus NAC to prevent CI-AKI.
REMEDIAL II was a multicenter, prospective trial that included of 294 patients at elevated risk of contrast nephropathy, randomized to either hydration by the RenalGuard™ system (n=135; hydration with normal saline [target urine flow ≥300 ml/h]+1.5 g/L NAC+ 0.25 mg/kg furosemide) or hydration with sodium bicarbonate and acetylcysteine (n=145; hydration by 3 ml/kg of IV sodium bicarbonate for 1 hour before treatment and 1 ml/kg for 6 hours after, and 1200 mg NAC bid x 2 and 1.5 g during therapy). In all cases, the contrast media that was administered was iodixanol (an iso-osmolar, nonionic contrast agent).
The primary endpoint was the rate of CI-AKI, defined as an increase of ≥0.3 mg/dL in serum creatinine (sCr) concentration 48 hours after the procedure. Secondary endpoints included an increase in the sCr concentration ≥25% and ≥0.5 mg/dL at 48 hours after contrast exposure; changes in serum cystatin C (sCyC) concentration at 24 and 48 hours after contrast exposure; the rate of acute renal failure that required dialysis; the rate of in-hospital and 1-month major adverse events (composite of death, renal failure requiring dialysis, or acute pulmonary edema); and changes in serum and urine NGAL concentrations at 2, 6, 12, 24, and 48 hours postcontrast exposure.
The mean age of the 294 patients was 75 years, about one-third were women, almost all had hypertension, 70% had diabetes, half were on an ACE inhibitor, and the mean eGFR was 32 ml/min/1.73 m2. The mean volume of contrast that was infused was about 135–140 ml.
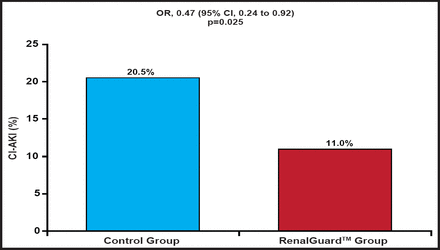
The percentage primary endpoint occurred in 11% in the RenalGuard™ group and 20.5% in the control group (OR, 0.47; 95% CI, 0.24 to 0.92; p=0.025; Figure 1). This translated into an absolute risk difference of 8.5%, or a number needed to treat of 12 to prevent 1 patient with a 10% rise in creatinine after contrast exposure. The secondary endpoint of an increase in the sCr concentration ≥25% at 48 hours after contrast exposure occurred in 2.7% in the RenalGuard™ group and 13% in the control group (p=0.001). Similarly, changes in creatinine and cystatin C at 48 hours (secondary endpoints) were significantly reduced (Table 1) in the RenalGuard™ group. At 1 month, 0.7% of patients in the RenalGuard™ group versus 4.8% in the control group needed dialysis (number needed to treat of 25 to prevent 1 patient requiring dialysis; p=0.031). The cumulative secondary endpoint of major adverse events occurred in 6.8% in the RenalGuard™ group compared with 9.6% in the control group (p=0.52)
Secondary Endpoints.
Primary Endpoint.
CI-AKI=Contrast-Induced Acute Kidney Failure
Data from REMEDIAL II demonstrate that among patients who are at high risk for contrast nephropathy, the aggressive hydration and matched fluid balanced that were achieved with the RenalGuard™ system (in conjunction with NAC and furosemide) were superior to hydration with sodium bicarbonate plus NAC in preventing contrast-induced sCr increases. Future trials should determine whether the automated closed-loop system that balances fluid management could be replicated simply with a routine aggressive hydration strategy.
- © 2011 MD Conference Express