Summary
Defining diabetes has become more complex than a simple classification of type 1 and type 2. As more is learned about the pathophysiology of the disease, subtle distinctions have been found, which have implications for diagnosis and treatment. Diabetes that is linked to another disease, or to organ transplantation, may also require a different management strategy. Latent autoimmune diabetes in adults and post-transplant diabetes are two types of so-called “special diabetes” that have become more recognized over the past few years, creating challenges both in appropriate diagnosis and treatment.
- Diabetes Mellitus
Defining diabetes has become more complex than a simple classification of type 1 and type 2. As more is learned about the pathophysiology of the disease, subtle distinctions have been found, which have implications for diagnosis and treatment. Diabetes that is linked to another disease, or to organ transplantation, may also require a different management strategy. Latent autoimmune diabetes in adults (LADA) and post-transplant diabetes are two types of so-called “special diabetes” that have become more recognized over the past few years, creating challenges both in appropriate diagnosis and treatment.
LADA
The concept of LADA began in the early 1970s, when a marker of the autoimmune process in type 1 diabetes mellitus (T1DM) was discovered, said Jerry P. Palmer, MD, Seattle VA Puget Sound Health Care System, Seattle, Washington, USA. LADA is phenotypic type 2 diabetes mellitus (T2DM) but with autoantibodies that are characteristic of T1DM.
“One cannot distinguish LADA from type 2 diabetes by looking at the patients; measurement of immune markers is required,” said Dr. Palmer.
Other characteristics that distinguish LADA from T2DM (antibody-negative) are early failure of sulfonylureas, more rapid decline in endogenous insulin secretion, and earlier need for insulin treatment. Several other names have been given to the disease, including type 1.5 diabetes and antibody-positive T2DM.
Patients with LADA test positively for at least one of the four antibodies that are commonly found in T1DM: islet cell autoantibodies (ICAs), autoantibodies to GAD, IA-2, or insulin. ICAs and GAD antibodies are also common in LADA, but both IA-2 and insulin autoantibodies are much less common in LADA than in T1DM. Some patients with phenotypic T2DM have been shown to have negative antibody testing at diagnosis but positive testing later. In contrast, some patients with positive antibodies have negative testing later. The pathophysiology of T2DM may include β-cell autoimmunity, and that autoimmunity may be transient.
Dr. Palmer discussed the importance of immune testing within the context of the increasing number of children (aged >18 years) who are being diagnosed with T2DM. In a study of children with new-onset diabetes, a large proportion of those who were diagnosed with T2DM (14 of 19) tested positively for ICAs [Brooks-Worrell BM et al. J Clin Endo Metab 2004]. Similarly, autoantibodies were positive in 11 of 16 children in whom the type of diabetes was classified as “indeterminate” at diagnosis.
Some studies have indicated that T-cell reactivity to islet antigens may be a better marker for diabetes. Dr. Palmer and colleagues found that the level of glucagon-stimulated C-peptide correlated more strongly with T-cell positivity than with antibody positivity [Goel A et al. Diabetes 2007]. Their data suggested that measuring T-cell responses to multiple islet proteins in patients with phenotypic type 2 diabetes improves the identification of patients with autoimmune diabetes and distinguishes those who have a more severe β-cell lesion compared with antibody assessment only.
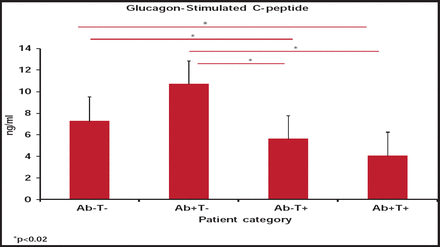
These findings were confirmed later, with a significantly lower level of glucagon-stimulated C-peptide found among adults with phenotypic T2DM who had negative antibody testing but T-cell positivity (p<0.02; Figure 1) [Brooks-Worrell BM et al. Diabetes Care 2011]. This diabetes variant of negative antibodies but T-cell reactivity represents a new classification of diabetes, one that cannot be detected through autoantibody testing alone.
Differences in the Level of Glucagon-Stimulated C-Peptide According to Antibody Testing and T-Cell Reactivity.
Reproduced with permission from J. Palmer, MD.
The term LADA is a misnomer, as the diabetes is not latent and can develop in children as well as adults. LADA, as a specific type of diabetes, may be inappropriate, Dr. Palmer added. The distinct separation of T1 and T2DM may need to be reevaluated.
Transplantation-Related Diabetes
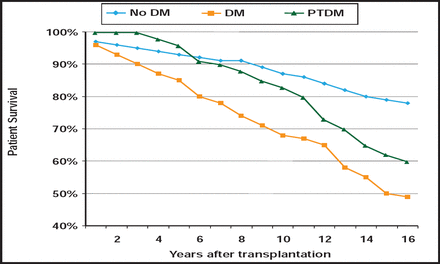
The development of diabetes after organ transplantation is becoming more prevalent due to the increasing number of transplantations, and the longer survival of both grafts and recipients. Post-transplant diabetes mellitus (PTDM) is particularly common after kidney transplant, but recognition of diabetes after liver and heart transplants is increasing, as more of these procedures are done. PTDM has “clear-cut consequences,” said Jennifer Larsen, MD, University of Nebraska Medical Center, Omaha, Nebraska, USA, with diabetes decreasing graft and patient survival after kidney transplant and increasing the risk of infection (Figure 2).
Impact of Diabetes on Patient Survival After Kidney Transplant.
Reproduced with permission from J. Larsen, MD.
International consensus guidelines for new-onset diabetes after kidney transplant were published in 2003 [Davidson J et al. Transplantation 2003]. The guidelines note criteria for PTDM that are the same as those established by the American Diabetes Association for diabetes in the general population; that is, a fasting blood sugar level of 126 mg/dL on two occasions, a random blood sugar level of 200 mg/dL or more with symptoms, and a 2-hour glucose level of 200 mg/dL or more during oral glucose tolerance testing.
The 2003 guidelines, however, have limitations. Because there is no standard for diabetes screening before transplantation, some patients who are identified with PTDM may have had undiagnosed diabetes before transplant. The criteria do not take into consideration the setting or timing of the hyperglycemia. Using these criteria, individuals who have just received high-dose corticosteroids could be identified as having PTDM, even if hyperglycemia resolves after the hospitalization. Likewise, individuals who develop diabetes 20 years after transplant could also be classified as having PTDM; yet, the implications are likely quite different. Perhaps the greatest limitation is that the guidelines have not been widely adopted across all transplant programs and groups.
There are many risk factors for PTDM. Many have preexisting risks, such as family history and obesity. History of hepatitis C infection also greatly increases risk after all types of organ transplant. After kidney transplant, individuals who receive a deceased donor graft are at higher risk for diabetes compared with those who receive a living donor graft. Risk varies with cause of renal failure, with those having hypertensive kidney disease and polycystic kidney disease at higher risk. There are several other candidate genes for risk after kidney transplant, including vitamin D receptor polymorphisms. A steatotic liver graft and a history of hepatitis or cirrhosis also increases risk after liver transplant. Finally, and most importantly, many of the immunosuppressant medications that are used for transplant also contribute to risk.
High-dose corticosteroids are associated with the highest risk for post-transplant diabetes. Other immunosuppressant agents also contribute to risk. The calcineurin inhibitors, tacrolimus more than cyclosporine, decrease insulin secretion and increase β-cell apoptosis. Sirolimus causes insulin resistance and later β-cell apoptosis as well. “However, the immunosuppression regimen should not be chosen with the hopes of avoiding diabetes. The primary goal of immunosuppression is to prevent rejection, because rejection [of the graft] has an even great impact,” Dr. Larsen said.
In PTDM, the diabetes goal is an HbA1C <7%. However, the approach to treatment is complex because of greater insulin resistance, changing glomerular filtration rate (GFR), frequent interruptions in eating, and drug-drug interactions. Metformin is contraindicated in most patients because of low GFR, frequent infections, and need for frequent contrast procedures. Sulfonylureas should be used only when the GFR is adequate because of the risk of hypoglycemia (>40 mL/min). Thiazolidinediones should be avoided after heart or liver transplantation. Exenatide should be avoided, because nausea can be more severe with renal insufficiency, and its impact on immunosuppressant drug absorption has not been well studied. In the end, most patients with PTDM will require insulin. As many transplant recipients have a lower GFR, their risk for hypoglycemia is higher; therefore short-acting insulins are preferable. Insulin should be tailored to the patient's eating pattern, and requirements can change rapidly.
Dr. Larsen emphasized the need for clinicians to evaluate the patient's feet, as neuropathy may not completely resolve and infections can develop much faster in the setting of immunosuppression. For this reason, patients should also be educated about the importance of foot care. Regular eye care is still important, because corticosteroids increase the risk of cataracts, and immunosuppression can increase the risk for eye infections as well.
Early recognition of PTDM leads to better control of HbA1C. Ideally, screening for diabetes should begin at the transplant evaluation and continue at least annually after transplant with fasting glucose and HbA1C. Dr. Larsen stressed the need for team management of PTDM.
- © 2011 MD Conference Express