Summary
This article discusses whether pharmacotherapy improves outcomes in patients with elevated triglycerides (TG) and normal low-density lipoprotein cholesterol. Limited data exist on the benefits of TG-lowering drugs on top of statins in high-risk patients.
- Lipid Disorders
Marja-Riitta Taskinen, MD, Helsinki University Hospital, Helsinki, Finland, discussed whether pharmacotherapy improves outcomes in patients with elevated triglycerides (TG) and normal low-density lipoprotein cholesterol (LDL-C). Limited data exist on the benefits of TG-lowering drugs on top of statins in high-risk patients.
A post hoc analysis of the PROVE IT-TIMI 22 trial, which randomized patients with acute coronary syndrome (ACS) to either intensive or standard statin therapy, showed that achievement of an on-treatment TG <150 mg/dL was independently associated with a lower risk of recurrent coronary heart disease (CHD) events, lending support to the concept that achieving low TG may be an additional consideration beyond low LDL-C in patients after ACS [Miller M. J Am Coll Cardiol 2008].
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, which randomized 9795 patients with type 2 diabetes mellitus (T2DM) and dyslipidemia to either fenofibrate or placebo, showed no reduction in the primary endpoint of coronary events with fenofibrate (p=0.16) [Keech A et al. Lancet 2005]. While the overall trial was neutral, there did appear to be benefit in an exploratory analysis of individuals with marked hypertriglyceridemia, especially among those with features of metabolic syndrome [Scott R. Diabetes Care 2009]. Sacks et al. [Sacks FM et al. N Engl J Med 2010] also found that fibrate treatment, combined with statin therapy, was not beneficial in reducing cardiovascular events compared with statin therapy alone in patients with T2DM in the primary results of the ACCORD trial. There was, however, a nonsignificant suggestion of heterogeneity in patients with high TG and low HDL (p-interaction 0.057).
Chapman et al. [Chapman MJ et al. Eur Heart J 2011] observed that even at the LDL-C goal, patients with cardiometabolic lipid abnormalities remain at a heightened risk of cardiovascular (CV) events. In the PROCAM study, about twice as many myocardial infarction (MI) survivors had elevated TG (200 mg/dL) and/or low HDL-C (<40 mg/dL) versus matched controls. This dyslipidemic profile was associated with increased CV risk, even in patients who achieved low LDL-C levels [Assmann G et al. Diab Vasc Dis Res 2010].
Prof. Taskinen noted that some high-risk individuals remain at a heightened risk of CV events even after achieving their LDL-C goal. Elevated TG, as a marker of triglyceride-rich lipoproteins (TRL) and their remnants, and low levels of HDL-C have been implicated in this residual excess CV risk. Prof Taskinen went on to note that elevated TG (≥150 mg/dL) and/or low HDL-C levels (<40 mg/dL) should be factors in considering further treatment [Chapman MJ et al. Eur Heart J 2011].
While there is observational and epidemiological evidence of an association between TG and outcomes, the benefits that have been observed with TG-specific therapies have been mixed and modest, especially in comparison with the proven benefits of statin therapy.
HDL – Is It a Treatment Target?
M. John Chapman, PhD, DSc, FESC, University Pierre and Marie Curie, Paris, France, discussed how both HDL quality and quantity may be important, noting that the role of HDL goes far beyond reverse cholesterol transport, and presented data on its antioxidant and anti-inflammatory properties.
Current observational evidence suggests a causal relationship between CV risk, elevated TRLs and their remnants, and low HDL-C [MJ Chapman. Eur Heart J 2011]. Wolfram et al. [Wolfram MR et al. Am J Cardiol 2006] found that patients with low HDL-C (<40 mg/dL in men; <45 mg/dL in women) are 3 times more likely to die after ACS (RR, 0.33; p=0.033).
While statin treatment reduces the risk of CV events in patients with low HDL, it does not abrogate low HDL-associated CV risk. Barter et al. [Barter PJ et al. N Engl J Med 2007] found that HDL-C levels predicted major CV events in patients who were treated with statins. Similarly, Sazonov et al. [Sazonov V. Atherosclerosis 2010] found that in statin-treated patients, while overall rates of adverse events were lower than in nonstatin-treated patients, elevated LDL-C, reduced HDL-C, and/or elevated TGs were still associated with a significantly increased relative risk of CV and/or cerebrovascular events compared with patients who had lipid parameters at target (HR, 1.24; 95% CI, 1.06 to 1.46; p=0.006).
The European Atherosclerosis Society Consensus Panel recently reviewed evidence for elevated TG-rich lipoproteins and low levels of HDL-C as CV risk factors [Chapman JM et al. Eur Heart J 2011]. They concluded that the data suggest that elevated TGs and their remnants, combined with low HDL-C, may play a causal role in premature coronary or peripheral atherosclerosis.
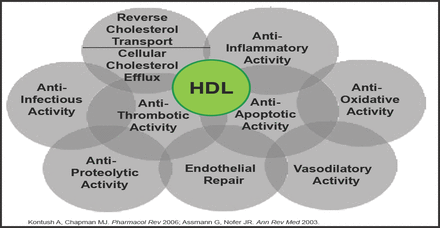
Recent applications of mass spectrometry-based proteomics have provided a new appreciation for the complexity of the HDL proteome by detecting more than 50 distinct HDL-associated proteins. Many proteins have known functions that do not fit easily into the randomized controlled trial paradigm (Figure 1) [Gordon SM et al. Trends Endocrinol Metab 2011].
Atheroprotective and Vasculoprotective Functions of HDL.
Reproduced with permission from M.J. Chapman, MD.
Findings link HDL to innate immunity and proteolytic pathways that are involved in inflammation and coagulation. The importance of these discoveries has been underscored by the demonstration that the proteomic profiles of HDL are altered in patients with CVD and can even be modified by lipid modification therapies [Gordon SM et al. Trends Endocrinol Metab 2011].
CETP Inhibition: Pros and Cons
Philip Barter, MD, PhD, The Heart Research Institute, Sydney, Australia, provided an update on a novel class of HDL-raising drugs, the cholesterol ester transfer protein (CETP) inhibitors.
Dr. Barter explained that CETP inhibition increases HDL-C and apoA-1 levels, decreases LDL-C and apoB levels, and decreases the content of very low-density lipoprotein by transferring cholesteryl esters from HDL to other lipoproteins [Barter P et al. Biochem Biophys Acta 1978]. It also plays a role in reverse cholesterol transport [Barter P. Biochem J 1982].
A meta-analysis of CETP genotypes and subjects with CHD showed polymorphisms that are associated with lower CETP mass and activity, have higher HDL-C levels and a significantly reduced coronary risk [Thompson A et al. JAMA 2008].
However, inhibiting CETP in humans with torcetrapib not only failed to reduce atherosclerosis in three imaging trials but also increased, rather than decreased, CV events in a large-scale endpoint trial (ILLUMINATE) [Barter P et al. N Engl J Med 2007]. Possible explanations for the adverse outcomes are that torcetrapib had an adverse off-target effect that was mediated through aldosterone that manifested in elevated blood pressure or that CETP inhibition, as a mechanism for raising HDL, may be harmful.
CETP drugs that are currently being assessed in large outcome trials include dalcetrapib and anacetrapib. The dal-OUTCOMES trial is assessing the efficacy and safety of dalcetrapib in 15,600 patients with recent ACS [Schwartz GG et al. Am Heart J 2009]. In the DEFINE trial, a Phase 2 safety trial of anacetrapib, treatment had no adverse effect on blood pressure, electrolytes, or aldosterone levels. The prespecified Bayesian analysis indicated that the event distribution in DEFINE provided a predictive probability (confidence) of 94% that anacetrapib would not be associated with the 25% increase in CV events that was seen in the torcetrapib trial [Cannon CP et al. N Engl J Med 2010]. The efficacy and safety of anacetrapib are being evaluated in a large CV outcome trial, called REVEAL.
According to Dr. Barter, these CETP drugs hold promise as an addition to the lipid-lowering arsenal, but it will take a few years for the results of clinical outcome trials to determine whether that promise can be realized in clinical settings.
Readers should note that while there is strong observational and epidemiological data that show an association between low HDL and adverse CV events, there is still debate as to whether HDL is an actual mediator of vascular events or whether it is a potent integrative risk marker. To date, there have been no large outcomes trials that have shown a benefit of HDL-raising therapy, particularly on a background of statin therapy.
Recently, the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes (AIM-HIGH) study, which randomized 3414 patients with cardiovascular disease, high TG, and low HDL to high-dose extended-release niacin or placebo on a background of statin therapy, was halted prematurely (18 months ahead of schedule), because niacin offered no benefit. In addition, there were numerically more strokes with niacin (28 vs 12). Additional exploratory analyses from this trial will be important to understand if there are specific subgroups that did benefit from niacin.
Overall, while observational data show an association between elevated TG and adverse outcomes, and low HDL and adverse outcomes, data that support clinical benefits of medications that target these lipid abnormalities remain mixed. Statin therapy that is titrated to LDL reduction goals remains the most critical component of lipid therapy for patients who are at risk of CV events. Results of large outcomes studies that use CETP inhibitors, which lead to robust increases in HDL as well as LDL reduction, will provide important new information.
- © 2011 MD Conference Express