Summary
From 1986 to 2002, sleep apnea affected >100 million individuals worldwide [WHO; Chronic Respiratory Diseases]. Apneas lead to periodic desaturation of O2 in arterial blood. In severe cases, O2 saturation drops to nearly 50%, and the frequency of apneas can reach as many as 60 to 90 episodes per hour. This article discusses the effects of intermittent hypoxia on glucose homeostasis.
- Sleep Disorders
- Insulin
- Diabetes Mellitus
- Renal Disease
From 1986 to 2002, sleep apnea affected >100 million individuals worldwide [WHO; Chronic Respiratory Diseases]. Recurrent apneas are characterized by transient cessation of breathing (approximately 10 to 30 seconds), due either to obstruction of the upper airways or to disturbances in respiratory rhythm generation (central apneas). Apneas lead to periodic desaturation of O2 in arterial blood. In severe cases, O2 saturation drops to nearly 50%, and the frequency of apneas can reach as many as 60 to 90 episodes per hour. Nanduri R. Prabhakar, PhD, University of Chicago, Chicago, Illinois, USA, discussed the effects of intermittent hypoxia on glucose homeostasis.
Sleep apnea is associated with myocardial infarctions, stroke, and other comorbidities, as well as altered glucose homeostasis and type 2 diabetes mellitus (T2DM). Despite a large body of evidence of epidemiological and clinical evidence that suggests that sleep-disordered breathing is an independent risk factor for the development of T2DM, the underlying pathogenesis of altered glucose metabolism in sleep apnea remains to be determined [Pallayova M et al. Med Hypotheses 2011].
Notwithstanding the previously proposed causal pathways that link sleep apnea with T2DM through increased insulin resistance and/or deterioration in insulin sensitivity, there is a paucity of information on sleep apnea-related alterations in pancreatic β-cell function [Palloyava M et al. Med Hypothesis 2011].
An examination of the effects of intermittent hypoxia on glucose homeostasis and pancreatic β-cell function in animal models showed that intermittent hypoxia increases basal plasma insulin levels but without a corresponding decrease in glucose levels, indicating insulin resistance. Hypoxia impairs glucose-stimulated insulin secretion as well. Evidence also suggests that hypoxia affects pancreatic β-cells, decreasing insulin content through insulin synthesis and/or processing of proinsulin to insulin. Intermittent hypoxia downregulates prohormone convertase 1, with an ensuing decrease in the processing of proinsulin to insulin [Palloyava M et al. Med Hypothesis 2011].
Considerable evidence indicates elevated reactive oxygen species (ROS) levels in patients who experience chronic intermittent hypoxia (CIH) is a consequence of recurrent apneas. The role of mitochondrial ROS in β-cell function is related to molecular mechanisms, including hypoxia-inducible transcription factor, with its constitutive and O2 subunits. Hypoxia-inducible factor 1 (HIF-1) mediates intermittent hypoxia-induced ROS generation via normoxia (NOX) upregulation [Prabhakar NR et al. Antioxid Redox Signal 2007].
Pathophysiological Role of Hypoxia in Diabetic Nephropathy
Chronic kidney disease (CKD) is a common complication of, and risk factor for, mortality for type 1 diabetes. The presence and severity of CKD remain the major determinants of excess mortality that is associated with type 1 diabetes.
Hypoxia is an important pathogenic factor in many renal diseases, including diabetic nephropathy. Deficiency of endogenous H2O may contribute to their pathogenesis by compromising medullary oxygenation [PH Groop et al. Diabetes 2009].
Aerobic exercise that is conducted during hemodialysis sessions contributes to the improvement of physical capacity and control of hypertension in patients with CKD. Data show a significant increase in the distance walked during the 6-minute walk test from 509±91.9 m to 555±105.8 m after exercise and a significant reduction in systolic blood pressure (151±18.4 mm Hg to 143±14.7 mm Hg), diastolic blood pressure (94±10.5 mm Hg to 91±9.6 mm Hg), and average arterial pressure (114±13.0 mm Hg to 109± 11.4 mm Hg) [Henrique DM et al. Arq Bras Cardiol 2010].
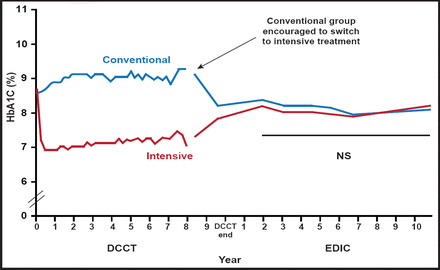
Intensive therapy effectively delays the onset and slows the progression of diabetic nephropathy in patients with type 1 diabetes [The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993]. The Diabetes Control and Complications Trial (DCCT) demonstrated the benefits of intensive treatment of diabetes in reducing glycemic levels and slowing the progression of diabetic nephropathy.
The DCCT cohort was examined annually for another 8 years as part of the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study. The persistent beneficial effects on albumin excretion and the reduced incidence of hypertension 7 to 8 years after the end of the DCCT suggest that previous intensive treatment of diabetes with near-normal glycemia during the DCCT had an extended benefit in delaying progression of diabetic nephropathy (Figure 1) [Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. JAMA 2003].
Persistent Beneficial Effects of EDIC/DCCT.
Reproduced with permission from PH Groop, MD.
Koh et al. [Nephrology (Carlton) 2011] found that both male and female diabetes subjects with microalbuminuria have elevated urine albumin-to-creatinine ratios (uACR) 1 hour postexercise (87.8, −24.3–199.4 & 6.7, 2.1–11.3). The authors concluded that exercise increased uACR estimation in normoalbuminuric subjects with diabetes, with a larger effect in females. It remains unknown whether exercise unmasks early diabetic nephropathy in normoalbuminuric subjects.
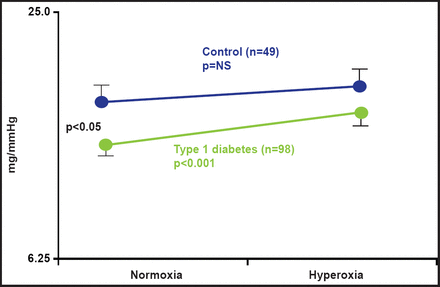
Blunted baroreflex sensitivity (BRS), typical of type 1 diabetes, is caused by a higher degree of tissue hypoxia in diabetes. Bernardi et al. [Diabetologia 2011] found that BRS increases after administration of oxygen in type 1 diabetes (p<0.05; Figure 2). The increased response to oxygen suggests a preexisting condition of tissue hypoxia that functionally restrains parasympathetic activity in patients with type 1 diabetes. Autonomic abnormalities can be partially or temporarily reversed by functional maneuvers, such as slow breathing, and oxygen administration through enhancement of parasympathetic activity and/or correction of tissue hypoxia.
BRS Increase After O2 Administration in Type 1Diabetes.
Reproduced with permission from PH Groop, MD.
Chronic hypoxia induces sequential abnormalities in oxygen metabolism in the kidneys of individuals with diabetes. Identification of these abnormalities improves our understanding of therapeutic benefits that can be achieved with antihypertensive agents, the control of hyperglycemia and/or hyperinsulinemia, and the dietary correction of obesity [Miyata T, de Strihou CY. Nat Rev Nephrol 2010]. According to Miyata and de Strihou, HIF has a key role in the body's defense against hypoxia. The activity of HIF is modulated by propyl hydroxylase 4—oxygen sensors whose inhibition may prove to be therapeutic.
In conclusion, Prof. Groop reminded attendees that patients with diabetes are hypoxic; the defense mechanisms to cope with hypoxia are impaired in diabetes; hypoxia leads to abnormalities in the autonomic and vascular functions that precede diabetic complications; regular exercise is a natural mode of treatment to cope with hypoxia; and there are new promising medications to fight hypoxia that are already under development.
- © 2011 MD Conference Express®