Summary
Cangrelor is a rapid-acting, reversible, intravenous ADP-P2Y12 receptor antagonist with a plasma half-life of 3 to 6 minutes. The Maintenance of Platelet Inhibition With Cangrelor After Discontinuation of Thienopyridines in Patients Undergoing Surgery [BRIDGE; NCT00767507] study results evaluated the use of cangrelor for bridging thienopyridine-treated patients to coronary artery bypass graft.
- Thrombotic Disorders
- Cardiology Clinical Trials
- Myocardial Infarction
- Interventional Techniques & Devices
Oral P2Y12 inhibitor therapy for up to 12 months is recommended following acute coronary syndrome (ACS) that is treated medically or after percutaneous coronary intervention (PCI). However, continuation of therapy puts patients at a 35% risk of bleeding, while preoperative discontinuation of antiplatelet therapy is associated with an increased risk of ischemic events. There is no currently established therapy with which a patient can be safely bridged during interruption of oral P2Y12 inhibitor therapy while the patient awaits surgery.
Cangrelor is a rapid-acting, reversible, intravenous (IV) ADP-P2Y12 receptor antagonist with a plasma half-life of 3 to 6 minutes. Dominick Angiolillo, MD, PhD, University of Florida-Shands, Jacksonville, Florida, USA, presented the Maintenance of Platelet Inhibition With Cangrelor After Discontinuation of Thienopyridines in Patients Undergoing Surgery (BRIDGE; NCT00767507) study results, which evaluated the use of cangrelor for bridging thienopyridine-treated patients to coronary artery bypass graft (CABG). The investigators hypothesized that cangrelor infusion would provide a level of platelet inhibition that was equivalent to that expected if oral thienopyridine was not discontinued. The objective of the study was to demonstrate that cangrelor would maintain levels of platelet reactivity <240 P2Y12 reaction units (PRUs).
The study was conducted in two stages. Stage I comprised the dose-finding phase of the study, in which the effective infusion dose of cangrelor was identified. For this stage, cangrelor was administered to cohorts of 5 patients at a time in a stepwise fashion at predetermined doses of 0.5 μg/kg/minute, 0.75 μg/kg/minute, 1.0 μg/kg/minute, and 1.5 μg/kg/minute until platelet inhibition was >60% in 80% of daily samples or a dose of 2.0 μg/kg/minute was reached.
In Stage II, patients with ACS or who were post-PCI on a thienopyridine and awaiting CABG were randomized to continuous IV cangrelor (n=106) or placebo (n=104) <72 hours after thienopyridine discontinuation. The patients received study treatment for at least 48 hours and up to 7 days, which was discontinued 1 to 6 hours before CABG. The primary endpoint was the percentage of patients with PRU <240, as measured by the VerifyNow P2Y12 test for all on-treatment samples. VerifyNow-P2Y12 is a rapid assay that tests platelet activity and directly measures the effects of a P2Y12 inhibitor on the P2Y12 receptor.
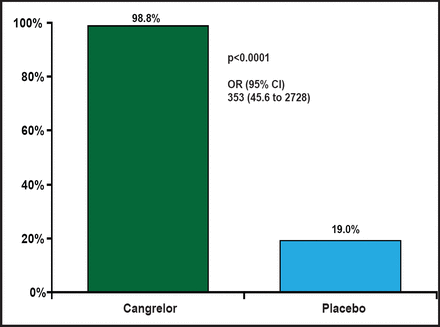
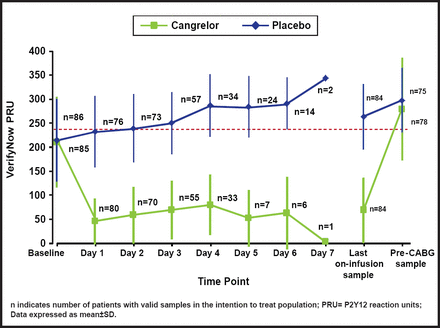
The percentage of patients with PRU <240 on all on-treatment samples was 98.8% in the cangrelor arm and 19.0% in the placebo arm (OR, 353; 95% CI, 45.6 to 2728; p<0.0001; Figure 1). A wide difference in platelet reactivity by day from baseline was observed between patients who were treated with cangrelor and those who were treated with placebo (Figure 2). On Day 1, PRU dropped from about 220 to 50 in patients who were treated with cangrelor and increased to 250 in those who were treated with placebo. Over the 7 days of treatment, PRU values in the cangrelor group ranged from 50 to 100, while PRU values in the placebo group continued to increase from 250 to about 350. There was a sharp increase in platelet reactivity from <100 PRU to >300 PRU that occurred within hours following discontinuation of IV cangrelor prior to CABG. This rapid increase in PRU is consistent with a quick dissipation of the antiplatelet effect of cangrelor prior to CABG.
Primary Endpoint.
Reproduced with permission from D. Angiolillo, MD, PhD.
Platelet Reactivity By Day.
Reproduced with permission from D. Angiolillo, MD, PhD.
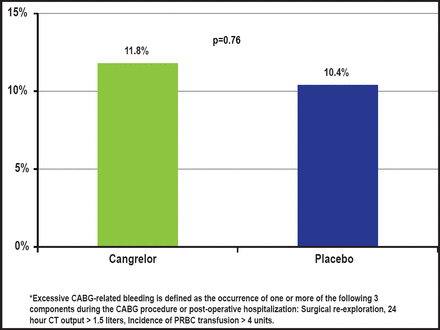
There was no significant difference in the rate of excessive CABG-related bleeding with cangrelor versus placebo (11.8% vs 10.4%; p=0.76 respectively; Figure 3). There were no fatal CABG-related bleeding events in either group. Similarly, there were no differences in pre-CABG major or minor bleeding, as defined by the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) scale; major bleeding rates were 2.8% with cangrelor versus 1.0% with placebo (p=0.358), and minor bleeding rates were 17.9% with cangrelor versus 9.9% with placebo (p=0.101).
Bleeding Endpoint.
Reproduced with permission from D. Angiolillo, MD, PhD.
The incidence of composite pre-CABG ischemic events (death, myocardial infarction, ischemia-driven target revascularization, or stroke) was 2.8% versus 4.0%. Death prior to CABG was 0.9% versus 3.0%, post-CABG ischemic events were 3.9% versus 4.2%, and death post-CABG was 1.0% versus 2.1%.
The results of the BRIDGE trial demonstrate that IV cangrelor rapidly achieves adequate platelet inhibition with a consistent effect during the infusion and rapid normalization of platelet function after discontinuation without increasing bleeding. The use of IV cangrelor as a bridge for patients who require prolonged dual antiplatelet therapy prior to cardiac surgery appears feasible. Larger trials are needed to more definitively establish and quantify the bleeding risk, safety profile, and anti-ischemic efficacy of cangrelor as a bridging therapy in patients with ACS or stents who are awaiting surgery.
- © 2011 MD Conference Express