Summary
Ziprasidone demonstrated efficacy and tolerability among patients with depressive mixed state, according to a recent study. In this double-blind, placebo-controlled, multicenter trial that included 74 patients who met DSM-IV criteria for major depressive disorder and two to three DSM-IV mania criteria, patients were randomized to receive either ziprasidone 40–160 mg/daily (n=36) or placebo (n=38) for 6 weeks in a forced titration regimen.
- Mood Disorders Clinical Trials
Ziprasidone demonstrated efficacy and tolerability among patients with depressive mixed state, according to a recent study by Ashwin Patkar, MD, Duke University Medical Center, Durham, NC, and colleagues.
In this double-blind, placebo-controlled, multicenter trial that included 74 patients who met DSM-IV criteria for major depressive disorder (MDD) and two to three DSM-IV mania criteria, patients were randomized to receive either ziprasidone 40–160 mg/daily (n=36) or placebo (n=38) for 6 weeks in a forced titration regimen. All patients had a baseline diagnosis of bipolar disorder type II (60%) or unipolar MDD (40%; n=30). Twenty-two percent of patients experienced rapid cycling (n=16), while most (78%) experienced nonrapid cycling (n=58). The majority of patients (52%; n=39) was not taking any psychotropic medication at baseline. However, 38% was taking antidepressants (n=28), 3% was taking mood stabilizers (n=2), and 7% was taking mood stabilizers plus antidepressants (n=5) at baseline. The primary outcome was change in Montgomery-Asberg Depression Rating Scale (MADRS) score from baseline to end of treatment. Secondary outcomes included Clinical Global Impression-Improvement (CGI-I) scores of 1 or 2, reduction in Clinical Global Impression-Severity (CGI-S) ≥1 from baseline to end of treatment, and change from baseline to end of treatment in Quick Inventory of Depressive Symptomatology (QIDS), Mania Rating Scale (MRS), Hamilton Anxiety Scale (HAM-A), or Global Assessment of Function (GAF).
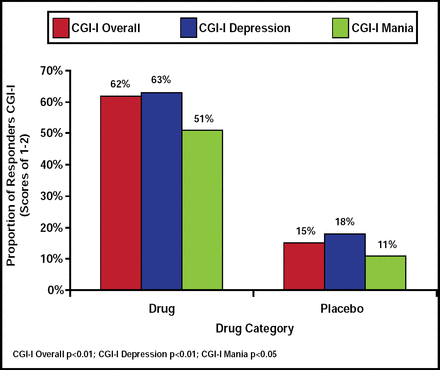
The ziprasidone group demonstrated significant improvement in MADRS score (23.19±6.18 at baseline to 10.73±9.91 at end of treatment) compared with placebo (24.73±8.03 at baseline to 19.35±8.42 at end of treatment; p<0.01), and no difference was associated with age, gender, race, rapid cycling, or diagnosis. Ziprasidone was found to be superior to placebo with regard to overall CGI-I (p<0.01), CGI-I depression (p<0.01), and CGI-I mania (p<0.05) scores (Figure 1). However, no significant drug superiority was associated with CGI-S overall or CGI-S mania (p<0.07) compared with placebo. GAF scores and QIDS favored ziprasidone over placebo (p<0.01 for both), though no significant difference was observed in MRS or HAM-A scales.
Proportion of Responders by CGI-I Score.
Reproduced with permission from A. Patkar, MD.
No serious adverse events were reported throughout the duration of the study. Changes in weight over the course of the study were similar for both groups. The most common adverse events that were noted in the ziprasidone group were insomnia (24%), irritability (23%), and dry mouth (20%; Table 1).
Adverse Events.
This study demonstrated that use of the antipsychotic ziprasidone for the treatment of the depressive mixed state is effective and well tolerated. Response to ziprasidone therapy did not appear to be associated with gender, race, age, diagnosis, or rapid cycling. Larger studies are needed to assess the clinical utility of ziprasidone and to establish the safety and efficacy of ziprasidone for the depressive mixed state, particularly with regard to ziprasidone as monotherapy.
- © 2010 MD Conference Express