Summary
Evidence from the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin [JUPITER; NCT00239681] trial [Ridker PM et al. N Engl J Med 2008] provides support for the use of statins to prevent strokes in healthy individuals with low LDL cholesterol but elevated high-sensitivity C-reactive protein levels.
- Neurology Clinical Trials
- Prevention & Screening
- Lipid Disorders
- Cerebrovascular Disease
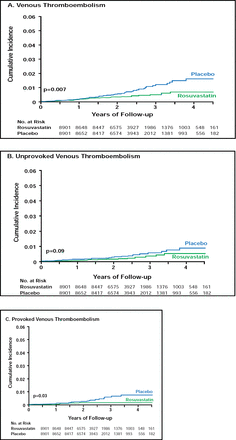
Evidence from the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER; NCT00239681) trial [Ridker PM et al. N Engl J Med 2008] provides support for the use of statins to prevent strokes in healthy individuals with low LDL cholesterol but elevated high-sensitivity C-reactive protein (hsCRP) levels. Robert Glynn, PhD, Brigham and Women's Hospital, Boston, MA, presented results that showed that rosuvastatin (20 mg/day) reduced the incidence of stroke by 48% after 1 year of treatment compared with placebo (Figure 1). The benefits occurred across all subgroups, including patients at higher risk, with no evidence of an increased risk for hemorrhagic stroke.
Cumulative Incidence of Venous Thromboembolism.
Copyright© 2009 Massachusetts Medical Society. All rights reserved.
Although lipid levels are significant risk determinants for ischemic stroke and coronary vascular disease (CVD), several studies have shown that individuals with high levels of hsCRP are at increased risk for stroke [Rost NS et al. Stroke 2001; Ballantyne CM et al. Arch Intern Med 2005; Everett B et al. JACC 2006]. The objective of this study was to determine if rosuvastatin would reduce stroke rates among individuals with low levels of cholesterol but elevated levels of hsCRP.
JUPITER enrolled 17,802 apparently healthy men and women at 1315 sites in 26 countries. Patients were required to have LDL levels <130 mg/dL and hsCRP levels >2.0 mL, be aged ≥50 years (men) or ≥60 years (women), and have no CVD or diabetes. The primary endpoint was the first major cardiovascular event (nonfatal myocardial infarction, nonfatal stroke, arterial revascularization, hospitalization for unstable angina, or cardiovascular death).
Median age of the study population was 66 years; 38% was women; 12% was black and 12% was Hispanic; mean blood pressure was 134/80 mm Hg; 16% smoked; and 17% used aspirin. After 12 months, rosuvastatin reduced LDL cholesterol levels by 50% (sustained for the course of the trial), hsCRP by 37% (sustained), triglycerides by 17%, and HDL by 4% compared with placebo.
The trial was stopped after a median follow-up of 1.9 years, because a significant (p<0.00001) change in the HR (0.56; 95% CI, 0.46 to 0.69) of the primary endpoint was reached. Overall, rosuvastatin was associated with a 44% reduction in the composite endpoint. There were 33 stroke events in the rosuvastatin group and 64 in the placebo group, a 48% reduction in HR (0.52; 95% CI, 0.34 to 0.79; p=0.002). For nonfatal stroke, the HR was 0.52 (95% CI, 0.33 to 0.80; p=0.003); ischemic stroke HR was 0.49 (95% CI, 0.30 to 0.81; p=0.004). There was no increase in the risk of hemorrhagic stroke (HR 0.67; 95% CI, 0.24 to 1.88; p=0.44). The secondary endpoint, all-cause mortality, decreased 20% with rosuvastatin (HR 0.80; 95% CI, 0.67 to 0.97; p=0.02).
These results were consistent across all subgroups with patients in high-risk groups (hypertension, smokers, high Framingham risk factors), showing substantial benefits from rosuvastatin treatment. Except for cancer deaths (higher in the placebo group) and the incidence of diabetes (higher in the rosuvastatin group), adverse events were similar between the treatment groups.
Compared with previous trials (WOSCOPS and AFCAPS), the JUPITER trial showed significant positive effects on stroke reduction. Dr. Glynn suggested that these benefits may be due to JUPITER's larger patient population, patient characteristics (more women and lower LDL levels in the JUPITER trial and higher smoking and lipid levels in the WOSCOPS trial), or the treatment (rosuvastatin in JUPITER, pravastatin in WOSCOPS, and lovastatin in AFCAPS).
- © 2009 MD Conference Express