Summary
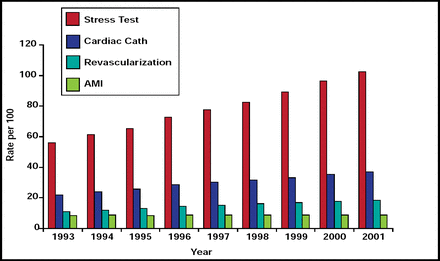
Although the rates of acute myocardial infarction (MI), revascularization, and cardiac catherization have remained relatively stable, the use of cardiac imaging has been increasing 6% per year. This article discusses several suggestions to manage this growth, hybrid and sequential imaging techniques, and whether improved imaging technology impacts clinical outcomes.
- Cardiac Imaging Techniques
- Imaging Modalities
- Tomography
Although the rates of acute myocardial infarction (MI), revascularization, and cardiac catherization have remained relatively stable, the use of cardiac imaging has been increasing 6% per year (Figure 1). Raymond J. Gibbons, MD, Mayo Clinic, Rochester, MN, said that physicians need to manage this growth. He made several suggestions that are supported by recent studies.
Medicare Cardiac Procedures.
Reproduced with permission by R. Gibbons.
-
Do not screen asymptomatic patients. Results of the DIAD (Detection of Ischemia in Asymptomatic Diabetics) study showed no difference in the cumulative incidence of cardiac events between patients who were screened with adenosine stress radionuclide myocardial perfusion imaging (MPI) and those who were not screened [Young LH et al. JAMA 2009].
-
Wait for solid evidence before using the new imaging tools.
-
Results from a study that was conducted by Dr. Gibbons's group showed that stress scores that were derived from SPECT (Single Photo Emission Computed Tomography) produced no increase in diagnostic yield in atrial fibrillation patients.
-
Do not use echocardiography as a tool to improve patient selection for cardiac resynchronization therapy (CRT). Data have shown a great deal of intra- and interobserver variability with this test [Chung ES et al. Circulation 2008].
-
-
Follow the available evidence. The American College of Cardiology/American Heart Association Angina Guidelines do not recommend the use of echocardiography in patients with a normal ECG, no history of MI, and no signs or symptoms that are suggestive of heart failure, valvular heart disease, or hypertrophic cardiomyopathy.
Finally Dr. Gibbons suggested that physicians need to change their perspective regarding the use of imaging technology. Despite the existence of guidelines, inappropriate testing with SPECT and echocardiography occurs 14% to 23% of the time. “It is not enough to have criteria for the use of imaging technology,” he said. “Reform must start with the physician.” The potential savings from not performing unneeded echocardiograms could amount to $1.5 billion/year [Gibbons RJ. Am Heart J 2000].
Professor Philipp A. Kaufmann, MD, University Hospital, Zurich, Switzerland, discussed hybrid and sequential imaging techniques.
The hallmark of hybrid imaging is the combined or fused imaging of two datasets in which both contribute equally to the image. Most often, it is a combination of nuclear techniques (positron emission tomography or SPECT) with computed tomography (CT). In patients with suspected coronary artery disease (CAD), SPECT and CT act synergistically and add significantly improved predictive value (p<0.005) [van Werkhoven JM et al. J Am Coll Cardiol 2009]. Combining the functional information of SPECT and the morphological information of CT may allow easier evaluation of the spatial relationship between coronary stenoses and perfusion defects. It may also provide added diagnostic information on the functional relevance of coronary artery lesions, particularly in patients with multivessel disease of intermediate lesion severity [Gaemperli O et al. J Nucl Med 2007].
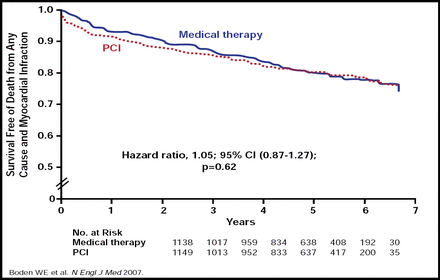
Incremental prognostic information that is derived from SPECT-MPI increases the yield of patients who are at high risk of cardiac death, and as shown in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, it may also reduce costs for intermediate-risk patients for whom noninvasive clinical testing and management strategies are sufficient. Results from COURAGE, which compared percutaneous coronary intervention (PCI) plus optimal medical therapy (OMT) with OMT alone in patients with stable CAD, showed no significant differences between PCI + OMT and OMT alone in the composite endpoint of death, MI, and stroke (20.0% vs 19.5%; HR, 1.05; 95% CI, 0.87 to 1.27; p=0.62) after 6 years (Figure 2) [Boden WE et al. N Engl J Med 2007].
Survival Free of Death (any cause) and MI.
Copyright © 2007 Massachusetts Medical Society. All rights reserved.
The addition of fractional flow reserve (FFR) to angiography may improve clinical outcomes in patients with multivessel CAD who are undergoing PCI with drug-eluting stents. Prof. Kaufmann reported the results from the recent FAME (Fractional Flow Reserve versus Angiography for Guiding PCI in Patients with Multivessel Coronary Artery Disease) trial, which showed that FFR plus angiographically guided PCI significantly (p=0.02) reduced the rate of the 1-year composite endpoint of death, nonfatal MI, and repeat revascularization [Tonino PA et al. N Engl J Med 2009].
Prof. Kaufmann recommends the use of the simplified Zurich algorithm, based on age, to determine the correct scan protocol.
Randolph P. Martin, MD, Emory University School of Medicine, Atlanta, GA, addressed the question of whether improved cardiac imaging technology has had any impact on clinical outcome.
Cardiovascular imaging is a target for cost savings in the current US health care debate and its growth may be related to a fear of lawsuits. “When assessing the value of a diagnostic test,” said Dr. Martin, “it can be argued that although tests are mostly approved because of their ability to rule in or out a disease, what really matters is the improved benefit of the test (and the cost) to the patient. Yet, there is little data that answers the question of whether these tests improve patient survival.”
Offering an example of inappropriate testing, he cited the use of imaging to evaluate syncope in older patients, noting that results from a study of 2106 patients aged 65 years or older who were hospitalized following a syncopal episode showed that cardiac enzyme tests, CT scans, echocardiography, carotid ultrasonography, and electroencephalography affected diagnosis or management in <5% of cases and helped determine the etiology of syncope <2% of the time, while postural blood pressure recording had the highest yield with respect to affecting diagnosis (18% to 26%) or management (25% to 30%) and determining etiology of the syncopal episode (15% to 21%) [Mendu ML et al. Arch Intern Med 2009].
Dr. Martin also reviewed a few of the studies that showed significant benefits from imaging. One of these was a European study in which cardiovascular magnetic resonance (CMR) was able to satisfy 86% of all imaging needs and impact management in nearly two-thirds of patients. Importantly, in 16% of cases the final diagnosis, based on CMR, was different from the diagnosis before CMR, leading to a complete change in management [Bruder O et al. J Am Coll Cardiol 2009].
Overall, Dr. Martin feels that while imaging is beneficial on an individual basis, “we still lack data.” Of the 745 recommendations for cardiovascular imaging in the new American College of Cardiology/American Heart Association guidelines, only 1% has a level of evidence of A. Future imaging trials must address patient outcomes that emphasize quality, value, and change in clinical course instead of sensitivity, specificity, and prognostic value.
- © 2009 MD Conference Express