Summary
This article discusses results from the Phase 2 dose-ranging RE-DEEM Trial, as well as results of a post hoc analysis of data from the RE-LY Trial.
- Cerebrovascular Disease
- Cerebrovascular Disease Clinical Trials
- Arrhythmias
- Myocardial Infarction
Results from the Phase II dose-ranging RE-DEEM trial (NCT00621855), presented by Jonas Oldgren, MD, Uppsala Clinical Research Center, Uppsala, Sweden, indicate that dabigatran up to 150 mg BID can be used in conjunction with dual antiplatelet therapy with only modestly increased bleeding risk.
RE-DEEM compared four dose regimens of dabigatran versus placebo in patients on dual antiplatelet therapy after acute coronary syndrome (ACS). The primary study endpoint was major (ISTH criteria) and clinically relevant minor bleeding. Secondary endpoints included coagulation activity and a composite of cardiovascular (CV) death, nonfatal myocardial infarction (MI), and nonhemorrhagic stroke.
Subjects (n=1878; mean age 61.8 years; 76% men) with ST or non-ST elevation ACS and ≥1 additional risk factor for CV complications who were already on dual antiplatelet therapy were randomly assigned to receive placebo or dabigatran 50 mg, 75 mg, 110 mg, or 150 mg BID for 6 months. The most common risk factors for CV complications were age ≥65 years (44%), diabetes (31%), previous MI (29%), and no revascularization for the index event (31%).
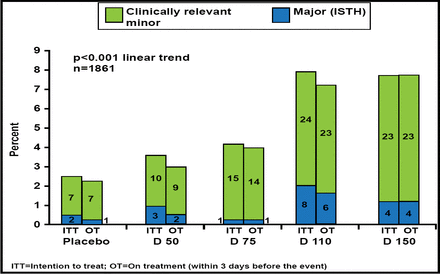
There was a significant dose-dependent (p<0.001) increase in the primary endpoint of major or clinically relevant minor bleeding (Figure 1). A comparison of major bleeding using the 3 major definitions showed a <1% increase with the highest dabigatran dose or with the two top doses combined (Table 1).
Primary Outcome: Bleeding.
Reproduced with permission by J. Oldgren, MD.
Major Bleeding Comparison.
Treatment with dabigatran doses resulted in a 45% reduction in D-dimer levels compared with placebo. There was a low rate of events for the composite secondary endpoint of CV death, nonfatal MI, and stroke. The study was not powered to show a difference between groups.
Dabigatran was well tolerated. Serious adverse events (AEs) were similar between the dabigatran doses and placebo, although slightly more dabigatran patients discontinued treatment, mostly due to bleeding. There was one fatal bleed in the placebo group and one in the 110 mg dabigatran group. There were no intracranial or intraspinal bleeds in any of the dose arms.
The investigators concluded that these results support the rationale for evaluating the 110- and 150-mg doses of dabigatran on clinical outcome in ACS in a larger study.
Lars Wallentin, MD, Uppsala Clinical Research Center, Uppsala, Sweden, presented the results of a post hoc analysis of data from the RE-LY trial (NCT00262600), showing that the reduction in the incidence of stroke and major bleeding in patients with atrial fibrillation (AF) that was seen in RE-LY was independent of the quality of INR control that was achieved at the individual study centers. For secondary outcomes, such as all vascular events and mortality, the advantage of dabigatran may be greater at centers with poorer INR control.
RE-LY was a prospective noninferiority trial that evaluated the safety and efficacy of dabigatran versus warfarin for stroke prevention in AF. Subjects were randomly assigned to open-label treatment with warfarin (INR 2.0 to 3.0; n=6022) or blinded treatment with dabigatran 110 mg BID (n=6076) or 150 mg BID (n=6015). Dabigatran 110 mg BID was shown to be noninferior to warfarin, and dabigatran 150 mg BID was superior to warfarin in reducing the incidence of stroke and systemic embolism (RR, 0.66; 95% CI, 0.53 to 0.82; p<0.001). There was no significant difference in the rate of major bleeding for dabigatran 150 mg BID versus warfarin (3.11% and 3.36% per year, respectively; p=0.31); the rate of major bleeding with dabigatran 110 mg BID (2.71% per year) was 20% lower versus warfarin (p=0.003) [Connolly SJ et al. N Engl J Med 2009].
This post hoc analysis was conducted to determine whether the dabigatran results were influenced by variations in the quality of INR control at the individual centers. The center average time in treatment range (TTR) in the warfarin arm was applied as a proxy for all patients at all centers and used to stratify patients into quartiles (<56.9%, 56.9% to 65.4%, 65.5% to 72.4%, and >72.4%). The primary endpoint was stroke or systemic embolism.
Results from the analysis were consistent with those from the overall study for the primary outcome and for the secondary outcomes of reduced intracranial and major bleeding, regardless of center TTR level. Indications of an interaction with center TTR level was seen for mortality, with dabigatran reducing mortality at centers with poor INR control but not those with good INR control. In the overall trial results, there was a significant reduction in all CV events (vascular events, death, and major bleeding) with dabigatran. In this analysis, these reductions appeared to be most relevant to those centers with poor INR control (Table 2).
Event Reductions.
Although acknowledging the limitations of the analysis, Prof. Wallentin concluded that these results appear to confirm the overall results of the RE-LY trial and provide additional information on how levels of INR control may influence outcomes.
- © 2009 MD Conference Express