Summary
Although coronary artery disease accounts for 75% of deaths in patients with diabetes, few studies have compared antidiabetic agents beyond their glucose-lowering efficacy. The prospective, randomized PERISCOPE trialtrial showed that an insulin-sensitizing drug (pioglitazone) may be more effective than a traditional insulin secretagogue (glimepiride) in stopping or reducing the progression of atherosclerosis.
- coronary artery disease clinical trials
- diabetes mellitus
Although coronary artery disease (CAD) accounts for 75% of deaths in patients with diabetes, few studies have compared antidiabetic agents beyond their glucose-lowering efficacy. A prospective, randomized trial showed that an insulin-sensitizing drug (pioglitazone) may be more effective than a traditional insulin secretagogue (glimepiride) in stopping or reducing the progression of atherosclerosis.
The PERISCOPE trial was a multicenter, double-blind trial that included 543 patients with CAD and type 2 diabetes. All patients had intravascular ultrasonography (IVUS) at study entry and were randomly assigned to treatment with glimepiride (1–4 mg) or pioglitazone (15–45 mg), with the drug titrated to the maximally tolerated dose by 16 weeks. At 18 months, a second IVUS examination was performed to determine the change in percent atheroma volume (PAV), the primary endpoint. Other IVUS endpoints included the mean maximum atheroma thickness, the total atheroma volume, and the atheroma volume in the most diseased 10-mm segment. Changes in biochemical parameters (levels of glycohemoglobin, insulin, and lipoproteins) and blood pressure were also evaluated.
Steven Nissen, MD, Cleveland Clinic, Cleveland, OH, reported that at 18 months, the change in PAV from baseline indicated highly significant progression of atherosclerosis among the patients treated with glimepiride (increase of 0.73%; p<0.001); in contrast, the PAV was essentially unchanged from the baseline (decrease of 0.16%; p=0.44) among patients treated with pioglitazone (Figure 1). The difference in the primary endpoint of the study between the two groups was highly significant (p=0.002). In contrast, there were no significant differences in the secondary IVUS endpoints between the two groups, with the exception of maximum atheroma thickness (increase of 0.011 mm for glimepiride vs decrease of 0.011 mm for pioglitazone; p=0.006).
Change in PAV (%).
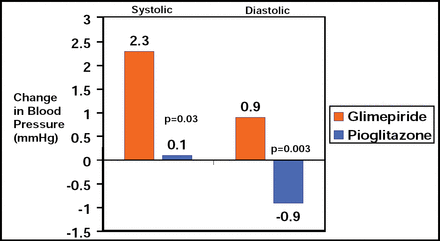
Mean (SD) baseline glycosylated hemoglobin levels were 7.4% (1.0%) in both groups and declined during treatment by an average of 0.55% (95% CI, −0.68% to −0.42%) with pioglitazone and 0.36% (95% CI, −0.48% to −0.24%) with glimepiride (between-groups p=0.03). Pioglitazone also had a greater effect on metabolic parameters, inflammation, and blood pressure (Table 1).
Comparison of Pioglitazone and Glimepiride on Biochemical Parameters and Blood Pressure.
With respect to safety, edema, fractures, and decreased hemoglobin levels occurred more frequently with pioglitazone, while hypoglycemia was more common with glimepiride.
Dr. Nissen compared the results of PERISCOPE with those of several other recent similar trials and noted that the collective findings suggest that glimepiride has a neutral effect on coronary disease progression. “However,” he added, “the pioglitazone group had substantially less progression than would have been predicted for the LDL level achieved, suggesting an anti-atherosclerotic effect.”
- © 2008 MD Conference Express