Summary
In previous studies, gefitinib combined with chemotherapy has provided no survival gain compared with chemotherapy alone for patients with advanced non-small cell lung cancer (NSCLC). However, a phase 3 trial has shown that sequential therapy with gefitinib may offer clinical benefit after platinum-based doublet chemotherapy in this setting. The West Japan Thoracic Oncology Group trial [WJTOG 0203; NCT00144066] involved patients who were randomly assigned to 3–6 cycles of chemotherapy alone (298 patients) or to 3 cycles of chemotherapy followed by daily gefitinib until disease progression (300 patients).
- Cancer
- Respiratory Cancers Clinical Trials
In previous studies, gefitinib combined with chemotherapy has provided no survival gain compared with chemotherapy alone for patients with advanced non-small cell lung cancer (NSCLC). However, a phase 3 trial has shown that sequential therapy with gefitinib may offer clinical benefit after platinum-based doublet chemotherapy in this setting.
The West Japan Thoracic Oncology Group trial (WJTOG 0203) (NCT00144066) involved patients who were randomly assigned to 3–6 cycles of chemotherapy alone (298 patients) or to 3 cycles of chemotherapy followed by daily gefitinib until disease progression (300 patients). The chemotherapy regimens consisted of cisplatin or carboplatin in combination with irinotecan, docetaxel, paclitaxel, vinorelbine, or gemcitabine. Most patients had adenocarcinoma (78% in the chemotherapy alone arm and 79% in the chemotherapy plus gefitinib arm), and most patients (82% overall) had stage IV disease.
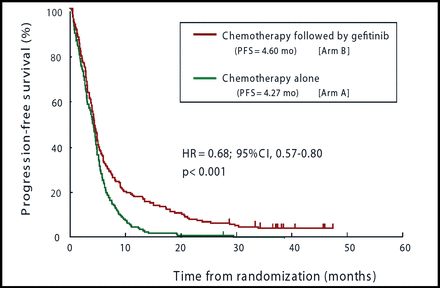
Toyoaki Hida, MD, PhD, Aichi Cancer Center, Nagoya, Japan, presented the findings on behalf of the WJTOG investigators. Dr. Hida reported that there was no significant difference between the two arms with respect to the primary endpoint of overall survival (OS). The mean survival was 13.68 months for the chemotherapy plus gefitinib arm and 12.89 months for the chemotherapy alone arm (p=0.10). However, progression-free survival (PFS; a secondary endpoint) was significantly longer for patients who received gefitinib (Figure 1).
Sequential Therapy With Gefitinib After Platinum-Based Doublet Chemotherapy Led to a Significantly Longer PFS Than Chemotherapy Alone for Patients With Advanced NSCLC.
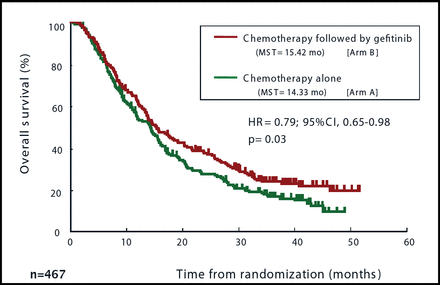
Dr. Hida added that a preplanned subset analysis showed that a platinum-based doublet followed by gefitinib was associated with a significantly superior OS for patients who had adenocarcinoma histology (Figure 2).
Gefitinib was Associated With Significantly Better OS Only for Patients With Advanced NSCLC of Adenocarcinoma Histology.
Chemotherapy followed by gefitinib was safe, with lower rates of adverse events than chemotherapy alone. Dr. Hida noted that the frequency of grade 3–4 anemia was significantly higher for the chemotherapy alone arm (21.8% vs 13.3%; p=0.006). All other toxicities were similar for the 2 arms. Among patients who received gefitinib, the 2 most common toxicities were elevated serum levels of liver enzymes (11.0%) and rash (4.1%).
Subgroup analysis showed that the hazard ratios (HRs) for death all favored sequential therapy with gefitinib except for non-adenocarcinoma histology (HR=1.24; 95% CI, 0.85–1.79) and stage IIIb disease (HR=0.99; 95% CI, 0.64–1.52).
Dr. Hida stated that in exploratory subgroup analyses, the mean survival was found to be longest for patients who had never smoked, with a slightly longer survival for patients in the chemotherapy alone arm (23.51 months vs 21.65 months; p=0.72). Among smokers, gefitinib led to significantly better survival (11.67 months vs 10.03 months; p=0.03); survival was further improved among smokers with adenocarcinoma histology (13.64 months vs 10.03 months; p=0.003).
- © 2008 MD Conference Express