Summary
Results from the Simvastatin and Ezetimibe in Aortic Stenosis [SEAS; NCT00092677] study indicate that intensive LDL-cholesterol-lowering with the combination of simvastatin 40 mg and ezetimibe 10 mg does not affect the progression of aortic valve stenosis, but can reduce the risk of cardiovascular ischemic events in subjects with mild-to-moderate asymptomatic aortic stenosis, when compared with placebo.
- valvular disease clinical trials
- lipid disorders
Results from the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis; NCT00092677) study indicate that intensive LDL-cholesterol (LDL-C)-lowering with the combination of simvastatin 40 mg and ezetimibe 10 mg does not affect the progression of aortic valve stenosis, but can reduce the risk of cardiovascular ischemic events in subjects with mild-to-moderate asymptomatic aortic stenosis (AS), when compared with placebo.
AS is a relatively common disease among elderly people and, if left untreated, can progress to death from heart failure or cardiac arrest. The standard treatment is valve replacement. There are no pharmacological therapies to prevent or treat this condition. Several studies have indicated that the cellular mechanism that is involved in the progression of AS may be similar to that of atherosclerosis [Rajamannan NM et al. Circulation 2002; 2003; 2005; Nat Clin Practi Cardiovasc Med 2007]; however, the results of one small prospective study that examined the effect of lipid-lowering on the progression of AS failed to find any effect [Cowell SJ et al. N Engl J Med 2005].
The objective of the SEAS study was to evaluate the effect of long-term, intensive cholesterol-lowering on clinical and echocardiographic outcomes in subjects with AS. The primary study endpoint was major cardiovascular events, a composite that consisted of death from cardiovascular causes, aortic valve replacement, congestive heart failure (CHF) resulting from the progression of AS, nonfatal myocardial infarction (MI), hospitalization for unstable angina, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), and nonhemorrhagic stroke. Key secondary outcomes included aortic valve events (eg, aortic valve replacement surgery, CHF due to aortic valve stenosis, or death from cardiovascular causes); ischemic events (death from cardiovascular causes, nonfatal MI, hospitalization for unstable angina, CABG, PCI, or hemorrhagic stroke), progression of AS as seen by echocardiography, and drug safety.
The study population included 1873 men and women aged 45 to 85 years (mean 67 years) with asymptomatic, echocardiographically confirmed mild-to-moderate aortic valve stenosis (mean aortic valve area of 1.28±0.47 cm2, with a mean and peak gradient of 23 and 39 mm Hg, respectively) and no other condition that was an indication for lipid-lowering therapy. After a diet run-in period of 4 weeks, subjects were randomly assigned to receive a combination of 40 mg simvastatin + 10 mg ezetimibe (n=944) or placebo (n=929). Subjects were followed for a minimum of 4 years; the median follow-up period was 52.2 months.
At Week 8, combination treatment with simvastatin/ezetimibe resulted in a 61% decrease from baseline LDL-C levels (140±36 mg/dL to 53±23 mg/dL) compared with no change in the placebo group.
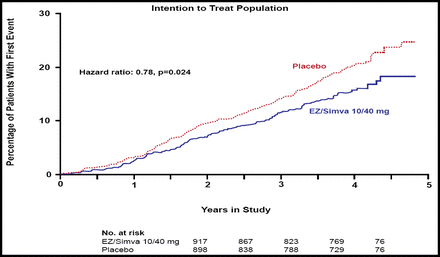
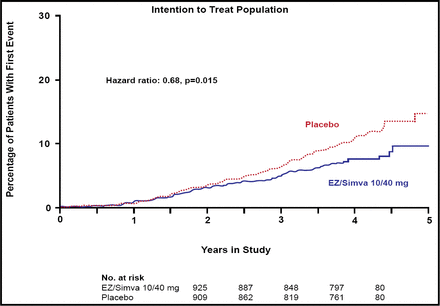
The SEAS study found no difference between the simvastatin/ezetimibe and placebo groups for the primary endpoint (HR 0.96; 95% CI, 0.83 to 1.12; p=0.59) or for the secondary outcome measures that were associated with aortic valve disease events (HR 0.97; 95% CI, 0.83 to 1.14; p=0.73). In contrast, significantly fewer subjects in the combination group experienced ischemic cardiovascular events versus those in the placebo group (148 [15.7%] vs 187 [20.1%]; HR 0.78, 95% CI, 0.63 to 0.97; p=0.024; Figure 1), a difference that primarily was driven by a lower incidence of CABG in the combination group (69 [7.3%] vs 100 [10.8%]; HR 0.68; 95% CI, 0.50 to 0.93; p=0.015; Figure 2). There was no difference between the two groups in any of the other components of the secondary endpoint.
Kaplan-Meier Curve for Secondary Outcome of Ischemic Cardiovascular Events.
CABG Intention to Treat Population: Percentage of Patients with First Event.
A significant difference was found in the incidence of cancer between treatment and placebo groups. Cancer was diagnosed in 11.1% (n=105) of subjects who received combination treatment versus 7.5% (n=70) subjects in the placebo group (p=0.01). Deaths from cancer also were more frequent in the ezetimibe/simvastatin group compared with placebo (39 [4.1%] vs 23 [2.5%], HR 1.67; 95% CI, 1.00 to 2.79; p=0.05). The cancers were not concentrated in any particular site.
The incidence of other serious adverse events was similar between the two groups, with the exception of liver enzymes. Significantly more subjects in the combination group had liver enzyme levels >3X ULN (16/925; 1.7%) versus in the placebo group (5/915; 0.5%; p=0.03).
In his discussion of the trial, Eugene Braunwald, MD, Brigham & Women's Hospital, Boston, MA, concluded that the SEAS study clearly demonstrated that the use of intensive lipid-lowering therapy with the combination simvastatin/ezetimibe does not prevent the progression of AS in patients with mild-to-moderate disease. A significant reduction of ischemic cardiovascular events was observed, however, which were mostly associated with reduced CABG. This reduction was seen as compared with placebo; there was no active control treatment arm. Dr. Braunwald felt that the finding of increased cancer in patients who were treated with simvastatin/ezetimibe was simply hypothesis-generating and commented that it was not confirmed by an analysis of data from 20,617 patients who were enrolled in the ongoing IMPROVE-IT (NCT00202878) and SHARP (NCT00125593) trials. This analysis did not show a significant excess risk of cancer in the active treatment (313 deaths) versus control (326 deaths) arm (risk ratio 0.96; 95% CI, 0.82 to 1.12; p=0.61) [Peto R et al. N Engl J Med 2008].
The editors would like to thank the many members of the ESC 2008 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2008 MD Conference Express