Summary
Treatment with investigative apixaban, an oral factor Xa inhibitor, shows promise as add-on protection against recurrent ischemic cardiovascular events among acute coronary syndrome patients who already are on standard antiplatelet therapy, including aspirin and clopidogrel. But dose-dependent bleeding remains an unresolved problem. This article discusses the Phase 2, Placebo-Controlled, Randomized, Double-Blind, Parallel Arm, Dose-Ranging Study to Evaluate Safety and Efficacy of Apixaban in Patients with a Recent Acute Coronary Syndrome [APPRAISE-1; NCT00313300] trial.
- cardiology clinical trials
- thrombotic disorders
Treatment with investigative apixaban, an oral factor Xa inhibitor, shows promise as add-on protection against recurrent ischemic cardiovascular (CV) events among acute coronary syndrome (ACS) patients who already are on standard antiplatelet therapy, including aspirin and clopidogrel. But dose-dependent bleeding remains an unresolved problem.
Investigators from the APPRAISE-1 (A Phase 2, Placebo-Controlled, Randomized, Double-Blind, Parallel Arm, Dose-Ranging Study to Evaluate Safety and Efficacy of Apixaban in Patients with a Recent Acute Coronary Syndrome; NCT00313300) trial reported their findings in Munich at the European Society of Cardiology Congress 2008.
“The addition of apixaban to standard antiplatelet therapy for 6 months after onset of ACS resulted both in dose-dependent increases in bleeding and in a trend toward a reduction in clinically important ischemic events,” said principal investigator John Alexander, MD, Duke Clinical Research Institute and Duke Heart Center, Durham, NC.
Dr. Alexander noted that one of the most challenging problems in treating ACS patients is finding a drug combination that inhibits clot formation without increasing the risk of serious bleeding. APPRAISE-1 is the first trial of an oral drug that targets factor Xa, a key enzyme in blood coagulation.
APPRAISE-1 was a phase 2 study that aimed at defining the optimal dose of apixaban regarding safety and efficacy in patients with a recent onset ACS. The study took place in 2 phases.
Phase A enrolled 547 patients who manifested ACS within the prior 7 days. Subjects were randomized to placebo (n=184), apixaban 2.5 mg BID (n=179), or apixaban 10 mg QD (n=184). Following the safety review of phase A, the enrollment was continued in phase B, reaching a study total of 1715 subjects. Those who were enrolled after the safety review were randomized to placebo (n=427), apixaban 2.5 mg BID (n=138), apixaban 10 mg QD (n=134), apixaban 10 mg BID (n=248), and apixaban 20 mg QD (n=221).
The primary safety outcome was major bleeding, as measured with the International Society of Thrombosis and Hemostasis (ISTH) scale, or clinically relevant non-major (CRNM) bleeding. The secondary efficacy outcome was a composite of CV death, myocardial infarction (MI), severe recurrent ischemia, and ischemic stroke.
During phase B of the trial, the higher apixaban dosing groups (10 mg BID and 20 mg QD) were discontinued due to unacceptably increased rates of total bleeding.
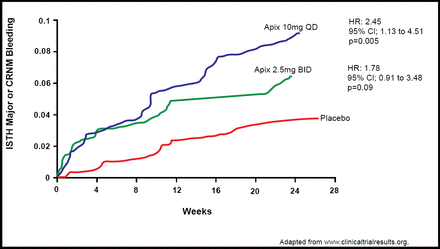
The index event was ST-elevation MI in 67% of patients. Investigators reported that the incidence of major or CRNM bleeding was 5.7% for apixaban 2.5 mg BID (n=315), 7.9% for apixaban 10 mg QD (n=315), and 3.0% for placebo (n=599). Bleeding at both apixaban dosages was higher compared with placebo (2.5 mg BID: HR 1.78; 95% CI, 0.91 to 3.48; p=0.09; and 10 mg QD: HR 2.45; 95% CI, 1.31 to 4.61; p=0.005; Figure 1). The absolute rates of bleeding were higher in patients on clopidogrel (7.0% for apixaban 2.5 mg BID, 9.1% for apixaban 10 mg QD, and 3.1% for placebo) compared with aspirin (2.4% for apixaban 2.5 mg BID, 4.1% for apixaban 10 mg QD, and 2.7% for placebo).
ISTH Major or CRNM Bleeding.
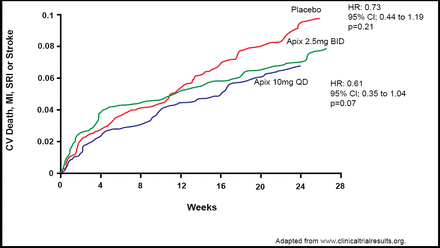
For the combined secondary efficacy endpoint outcome of CV death, MI, severe recurrent ischemia, or ischemic stroke, investigators reported incidence rates of 7.6% for apixaban 2.5 mg BID (n=317), 8.7% for apixaban 10 mg QD (n=318), and 6.0% for placebo (n=611). These findings suggested a trend toward efficacy versus placebo (2.5 mg BID: HR 0.73; 95% CI, 0.44 to 1.19; p=0.21; and 10 mg QD: HR 0.61; 95% CI, 0.35 to 1.04; p=0.07), but the difference did not reach statistical significance (Figure 2).
Ischemic Outcome.
Dr. Alexander said that while the clinical findings in APPRAISE-1 were inconclusive regarding efficacy, apixaban at 5 mg and 10 mg daily appears to be promising for ACS patients and warrants further clinical investigation in large, well-controlled trials.
- © 2008 MD Conference Express