Summary
A feasibility study has provided new insight on the value of therapeutic anticoagulation for isolated distal deep vein thrombosis (IDDVT). This pilot study reported a nonsignificant trend toward reduction of serious complications, including the absence of major bleeding, with anticoagulation. The Anticoagulation of Calf Thrombosis trial [ACT; ISRCTN75175695] aimed to compare therapeutic anticoagulation against conservative management for patients with acute symptomatic IDDVT.
- Thrombophilia
- Thromboembolic Disease
- Thrombotic Disorders
- Hematology Clinical Trials
- Hematology
- Thrombophilia
- Thromboembolic Disease
- Thrombotic Disorders
- Hematology Clinical Trials
A feasibility study has provided new insight on the value of therapeutic anticoagulation for isolated distal deep vein thrombosis (IDDVT). This pilot study reported a nonsignificant trend toward reduction of serious complications, including the absence of major bleeding, with anticoagulation. Daniel Horner, MD, Central Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom, reported the results of the study.
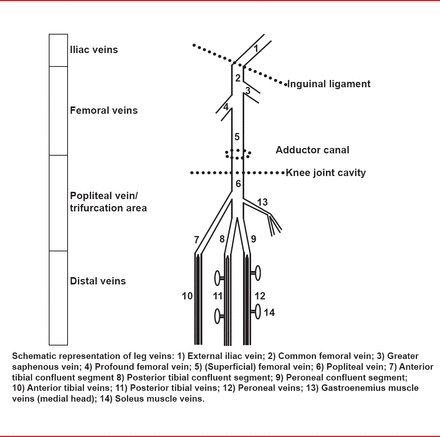
Half of all lower limb deep vein thrombi are located in the distal (calf) veins (Figure 1) [Palareti G, Schellong S. J Thromb Haemost 2012].
Diagram of the Distal (Calf) Vein
Reproduced from Palareti, G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing : Isolated distal DVT. J Thromb Haemost 2012;10(1)11–19. With permission from the International Society of Thrombosis and Haemostasis.
There is currently little evidence defining the clinical importance of detecting and treating IDDVT. Contemporary international guidelines vary regarding diagnostic and therapeutic advice. One meta-analysis suggested that anticoagulation therapy for IDDVT may decrease the rate of subsequent pulmonary embolism and thrombus propagation, but findings were not robust [De Martino RR et al. J Vase Surg 2012]. Thus, the risk and benefits of anticoagulation therapy remain poorly defined.
The Anticoagulation of Calf Thrombosis trial [ACT; ISRCTN75175695] aimed to compare therapeutic anticoagulation against conservative management for patients with acute symptomatic IDDVT.
The ACT study was a pragmatic, open-label, randomized controlled trial within a modern healthcare framework conducted to determine whether patients with IDDVT benefit from therapeutic anticoagulation. Principal feasibility outcomes included incidence of the index condition, recruitment rate and attrition (including loss to follow-up, protocol violation, and allocation crossover). A composite of thrombus propagation to the popliteal vein, symptomatic pulmonary embolism, death attributable to venous thromboembolic disease, or major bleeding was the primary clinical outcome. Propagation to any site, symptomatic progression, and minor or nuisance bleeding rates were secondary clinical outcomes.
Symptomatic IDDVT patients within a single ambulatory thrombosis center were included in the study. Participants were randomized to receive either phased therapeutic anticoagulation intervention (n=35) or conservative management (n=35). Both groups received Grade 2 compression stockings and analgesia. All patients underwent assessor-blinded, color-duplex sonographic imaging after 7 and 21 days, and follow-up at 3 months. Analysis was by intention-to-treat.
All predefined feasibility outcomes were achieved (Table 1).
Predefined Feasibility Criteria
The primary clinical outcome occurred in 4 of the patients in the control group and no one in the intervention group (absolute risk reduction [ARR], 11.4%; Table 2). The number-needed-to-treat (NNT) was 9. There were no cases of major bleeding in either group. Minor bleeding occurred in 3 (8.6%) patients in the control and 7 (20.0%) in the intervention groups (p=0.31). Allocation crossover occurred in 15 (21.4%) patients.
Composite Outcomes with Individualized Components
Propagation to any site (as recorded by an increasing Marder score at any stage during 3-month follow-up) occurred in 11 (31.4%) control patients compared with 2 (5.7%) intervention patients (ARR 25.7%; 95% CI, 5.9 to 44.3; p=0.01; NNT, 4). There was an increased rate of resolution in the intervention group but it was not significant (p=0.3).
The results of this study indicate that further primary research regarding IDDVT is feasible within a modern healthcare framework. The point estimate for the ARR in serious thromboembolic complications with therapeutic anticoagulation is roughly 11%. Limitations of the study include the use of sonography instead of contrast venography, a relatively short endpoint, and being a single-center study. The results of this pilot study will be used to plan a more definitive multicenter trial.
- © 2013 MD Conference Express®