Summary
A new meta-analysis suggests that dipeptidyl peptidase-4 (DPP-4) inhibitors are associated with a reduced risk of major cardiovascular events compared with placebo or other treatment, irrespective of trial duration, type of DPP-4 inhibitor, or comparator.
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
A new meta-analysis suggests that dipeptidyl peptidase-4 (DPP-4) inhibitors are associated with a reduced risk of major cardiovascular (CV) events compared with placebo or other treatment, irrespective of trial duration, type of DPP-4 inhibitor, or comparator. Edoardo Mannucci, MD, Careggi Teaching Hospital, Florence, Italy, presented findings from the study.
The aim of the meta-analysis was to assess the effect of DPP-4 inhibitors on the incidence of major CV events. The study included data from a total of 53 randomized clinical trials of “gliptin” drugs with a duration of at least 24 weeks (the search terms were sitagliptin, saxagliptin, alogliptin, linagliptin, vildagliptin, and dutogliptin), with more than 33,000 patients with type 2 diabetes who were randomized to a DPP-4 inhibitor, another diabetes drug, or placebo. Information on major CV events was available for a majority of these trials.
In all, 257 major adverse CV events occurred in the trials over a mean of 10 months: 137 in patients who took a DPP-4 inhibitor and 120 in those who took either placebo or another diabetes drug.
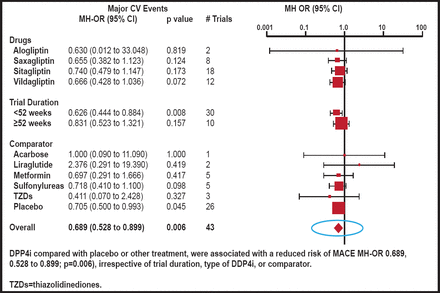
An intention-to-treat analysis that excluded trials in which there were no events, showed a reduced risk of major CV events (OR, 0.689; 95% CI, 0.528 to 0.899; p=0.006) for patients who were treated with a DPP-4 inhibitor compared with placebo or another diabetes agent (Figure 1). Outcomes were independent of trial duration, type of DPP-4 inhibitor, or comparator.
Odds Ratios for Major CV Events.
Reproduced with permission from E. Mannucci, MD.
In trials with follow-ups that ranged from 24 to 51 weeks, the use of DPP-4 inhibitors was associated with a significant reduction in major CV events. For trials that were ≥52 weeks in duration, the findings were not statistically significant. Prof. Mannucci reported that the point estimate for the odds ratio was approximately 0.7 for both shorter- and longer-term trials.
According to Prof. Mannucci, the observation of a significantly lower risk of major CV events in patients who were treated with DPP-4 inhibitors for a short time was unexpected. The outcome suggests the possibility of some unconventional CV protective effect that only requires a short incubation period. Possibilities include direct myocardial effects of GLP-1 receptor stimulation, ischemic preconditioning, a direct endothelial effect, and recruitment of endothelial cell progenitors.
Study limitations include the fact that CV events were not the principal endpoints of the included trials but were reported only as adverse events. In addition, the short duration of available trials does not allow for any inference on the long-term effects of treatment with DPP-4 inhibitors.
Based on outcomes, the authors concluded that the meta-analysis suggests a possible protective effect from CV events for patients who are being treated with DPP-4 inhibitors. This finding is in line with results from previously reported meta-analyses that are based on patient-level data. The possibility of an unconventional CV protective effect is further supported by the fact that shorter-term trials still yielded significant results.
Prof. Mannucci said that the meta-analysis clearly shows short- and medium-term CV safety with the use of DPP-4 inhibitors for the treatment of type 2 diabetes. He cited the need for specific trials on CV outcomes to have complete information on the effects of long-term exposure.
- © 2011 MD Conference Express®