Summary
The glucagon receptor antagonist LY2409021 (LY) substantially lowers HbA1C without severe hypoglycemia or weight gain in type 2 diabetes mellitus (T2DM) patients. In a double-blind, randomized, placebo-controlled, Phase 2 study researchers examined the margin between LY efficacy and safety by comparing mean changes in HbA1C and liver aminotransferases at 3 dose levels.
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
The glucagon receptor antagonist LY2409021 (LY) substantially lowers HbA1C without severe hypoglycemia or weight gain in type 2 diabetes mellitus (T2DM) patients. In a double-blind, randomized, placebo-controlled, Phase 2 study presented by Christof M. Kazda, MD, Eli Lilly and Company, Suresnes, France, researchers examined the margin between LY efficacy and safety by comparing mean changes in HbA1C and liver aminotransferases at 3 dose levels.
T2DM pathophysiology is characterized by greater postprandial glucose release, impaired insulin secretion, and abnormal glucagon plasma levels [Woerle HJ et al. Am J Physiol Endocrinol Metab 2006]. LY is a potent, selective glucagon receptor antagonist that inhibits hepatic glucose output and has significant glucose-lowering effects [Kelly RP et al. ADA 2011 Abstract 1004-P; Tham LS et al. ADA 2011 Abstract 416-PP]. In a Phase 1 study [NCT01606397], LY improved glycemic parameters and showed reversible dose-dependent increases in serum aminotransferase levels. The incidence of hypoglycemia was infrequent and was considered to be of mild to moderate intensity [Kelly RP et al. ADA 2011 Abstract 305-OR].
The primary endpoint of the current Phase 2a study [NCT00871572] was mean change in HbA1C and liver aminotransferases. Secondary objectives included the evaluation of LY effects on blood glucose, insulin, glucagon, glucagon-like peptide-1 (GLP-1), and blood lipids, as well as safety and tolerability.
Patients aged 18 to 70 years with T2DM (HbA1C 6.5% to 10%) and a body mass index of 25 to 40 kg/m2 who were treated with diet and exercise alone, or with a stable dose of metformin (≥1000 mg/day for ≥3 months prior to screening) were eligible. Key exclusion criteria were related to hepatic diseases. Subjects (mean age 50 years, mean diabetes duration between 3 and 5 years) were randomly assigned to LY 10 mg (n=17), 30 mg (n=34), 60 mg (n=26), or placebo (n=10) QD for 12 weeks. There were 52.9% to 58.8% of the LY patients taking metformin versus 70% of placebo subjects. Mean baseline HbA1C was highest in the LY 10-mg group (8%) and 7.5%, 7.6%, and 7.8% in the 30-mg, 60-mg, and placebo groups, respectively.
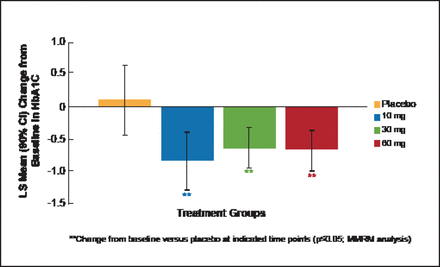
At Week 12, HbA1C change from baseline showed that LY was associated with significant (p≤0.05) dose-dependent improvements in glycemic control in contrast to placebo (Figure 1). LY also produced dose-dependent increases in fasting glucagon, alanine aminotransferase, aspartate aminotransferase, and total GLP-1 that returned to baseline after LY washout.
miTT Population: Mean Change from Baseline in HbA1C at Week 12.
LS=least squares; miTT=modified intention-to-treat; MMRM=Mixed Model Repeated Measures.
Reproduced with permission from CM Kazda, MD.
The proportions of patients who experienced any treatment-emergent adverse event (TEAE) were similar in all treatment groups. No severe TEAEs were reported. Two non-drug-related serious adverse events occurred. There were no severe hypoglycemia events and 4 confirmed (blood glucose measurements) hypoglycemia events. Incidence of hypoglycemia was not dose dependent. There were no significant changes from baseline observed in any of the 3 LY treatment groups compared with placebo for body weight, blood pressure, triglycerides, low-density lipoprotein and high-density lipoprotein cholesterol, bilirubin, fasting insulin, or active GLP-1.
Treatment with LY resulted in dose-dependent transient increases in mean aminotransferase, fasting glucagon, and total GLP-1 levels without elevated bilirubin or other signs/symptoms of liver injury. There were no increases in body weight, lipids, or blood pressure. The efficacy, safety, and tolerability profile of LY in patients with T2DM supports further clinical development.
The editors would like to thank the many members of the EASD Congress 2012 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®