Summary
Bisphenol A (BPA) is an industrial chemical used to make polycarbonate—a hard, clear plastic used in many consumer products. Endocrine-disrupting effects of low BPA doses in the microgram range are a matter of controversy. However, this article reports that an injection of a very low dose (25 ng/kg/day) of BPA into neonatal rats delayed puberty, slowed down gonadotropin-releasing hormone secretion, and changed hypothalamic RNA expression.
- diabetes & endocrinology clinical trials
- hormone therapy
Bisphenol A (BPA) is an industrial chemical used to make polycarbonate—a hard, clear plastic used in many consumer products. It is also found in epoxy resins, which act as a protective lining inside some metal-based food and beverage cans. Endocrine-disrupting effects of low BPA doses in the microgram range are a matter of controversy. In a late-breaking presentation, Jean-Pierre Bourguignon, MD, PhD, University of Liège, Liège, Belgium, reported that injection of a very low dose (25 ng/kg/day) of BPA into neonatal rats delayed puberty, slowed down gonadotropin-releasing hormone (GnRH) secretion, and changed hypothalamic RNA expression. Opposing effects, including early puberty, were observed with a high dose (5 mg/kg/day).
A related study reported on the effects of neonatal exposure to diethylstilbestrol (DES) on pubertal timing in female rats, showing that age at vaginal opening (VO) was advanced after exposure to 10 µg/kg/day of DES and delayed after 1 µg/kg/day, given subcutaneously [Franssen D et al. Reprod Toxicol 2014]. There were also consistent changes in maturation of pulsatile GnRH secretion.
In the current study, newborn female rats were exposed to vehicle (corn oil) or BPA, injected subcutaneously from postnatal day (PND) 1 to 5 or from PND 1 to 15. VO and estrous cyclicity were followed. The GnRH interpulse was studied ex vivo with hypothalamic explants obtained at PND 15, 20, or 25. Gene expression in the retrochiasmatic hypothalamus was assessed by whole-exome RNA sequencing on PND 20 (3 samples per condition).
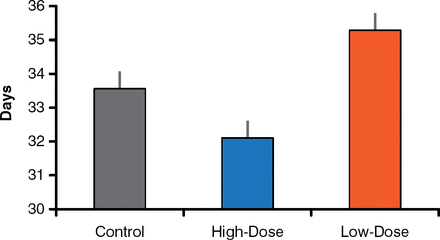
The age at VO after neonatal exposure to 25 ng/kg/day of BPA for 15 days was 35.3 ± 0.7 days, compared with 32.1 ± 0.6 days at 5 mg/kg/day. In animals receiving vehicle (control), VO was 33.5 ± 0.5 days. Thus, the high dose advanced, and the low dose delayed, the age at VO compared with the control (Figure 1). The difference in pubertal timing between the 2 doses was significant (p < .05).
Median Age at Vaginal Opening After High and Low Doses of BPA
BPA=bisphenol A.
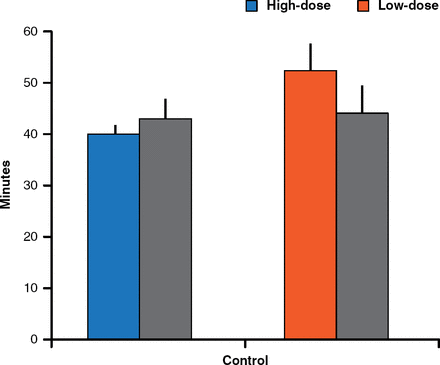
Late VO came after exposure to 25 ng/kg/day of BPA and was preceded at PND 20 by a significant increase in GnRH interpulse interval (52.5 ± 0.8 min vs 44.6 ± 0.7 min in controls). Early VO after exposure to 5 mg/kg/day, however, was preceded by a significant decrease in GnRH interpulse interval (40.3 ± 0.1 min vs 42.8 ± 0.4 min; Figure 2). After BPA exposure from PND 1 to 5, comparable dose-related changes in GnRH secretion were observed.
GnRH Interpulse Interval After Exposure to High and Low Doses of BPA
BPA=bisphenol A; GnRH=gonadotropin-releasing hormone.
RNA expression of 10 genes showed significant opposing changes in the high- versus low-dose groups at PND 20. The dose of 25 ng/kg affected expression of 14 genes, while 5 mg/kg modified the expression of 472 genes versus controls. A significant difference in levels of RNA expression was observed for 1407 genes when the 2 BPA dose conditions were compared.
Neonatal exposure to a very low dose of BPA delayed pubertal onset, slowed GnRH secretion, and changed hypothalamic RNA expression. Changed hypothalamic RNA expression confirmed the neuroendocrine effects of the 2 BPA doses, with opposing changes of similar genes in relation to BPA dose and with alteration of distinct genes by each dose.
While these data add to the large body of evidence in animal models concerning the effects of BPA on the female reproductive tract [Caserta et al. Reprod Biol Endocrinol 2014], the influence of BPA on the pathogenesis of premature puberty in girls has not been confirmed. Some have found a correlation between pubertal stages and BPA serum or urinary levels [Qiao et al. Wei Sheng Yan Jiu 2010; Durmaz et al. J Clin Res Pediatr Endocrinol 2014], while others have found conflicting data [Wolff et al. Environ Res 2008; Yum et al. J Environ Sci Health A Tox Hazard Subst Environ Eng 2013] perhaps due to confounding variables, such as body mass index and race [Wolff et al. Environ Health Perspect 2007]. More studies need to be conducted in humans.
- © 2014 MD Conference Express®