Summary
Although 2 studies [Stone GW et al. N Engl J Med. 2008; Steg PG et al. N Engl J Med. 2013] have documented the superiority of bivalirudin over heparin in patients with acute myocardial infarction (AMI) undergoing primary percutaneous coronary intervention (PCI), a third study showed heparin to be associated with fewer major adverse ischemic events with no increase in bleeding [Shahzad A et al. Lancet. 2014]. This article presents the results of the Bivalirudin in Acute Myocardial Infarction vs Glycoprotein IIb/IIIa and Heparin trial [BRIGHT; NCT01696110] in which bivalirudin monotherapy led to better 30-day and 1-year outcomes in these patients compared with heparin monotherapy or heparin plus tirofiban.
- Cardiology Clinical Trials
- Myocardial Infarction
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Myocardial Infarction
- Interventional Techniques & Devices
- Cardiology
Although 2 studies [Stone GW et al. N Engl J Med. 2008; Steg PG et al. N Engl J Med. 2013] have documented the superiority of bivalirudin over heparin in patients with acute myocardial infarction (AMI) undergoing primary percutaneous coronary intervention (PCI), a third study showed heparin to be associated with fewer major adverse ischemic events with no increase in bleeding [Shahzad A et al. Lancet. 2014]. Yaling Han, MD, Department of Cardiology, General Hospital of Shenyang Military Region, Shenyang, China, presented the results of the Bivalirudin in Acute Myocardial Infarction vs Glycoprotein IIb/IIIa and Heparin trial [BRIGHT; NCT01696110] in which bivalirudin monotherapy led to better 30-day and 1-year outcomes in these patients compared with heparin monotherapy or heparin plus tirofiban.
Patients aged 18 to 80 years, with STEMI within 12 hours of symptom onset, NSTEMI within 72 hours of symptom onset, and planned emergency PCI were eligible for enrollment. Patients with thrombolysis within 72 hours, cardiogenic shock, or anticoagulant use 48 hours before randomization, and those with active bleeding/bleeding diathesis, hemoglobin < 100 g/L, platelet count < 100 × 109/L, or creatinine clearance < 30 mL/min were excluded. Eligible subjects were randomized to bivalirudin (n = 735; 0.75 mg/kg bolus plus 1.75 mg/kg/h infusion followed by prolonged post PCI infusion for at least 30 minutes), heparin (n = 729; 100 U/kg bolus), or heparin plus tirofiban (n = 730; heparin 60 U/kg bolus and tirofiban 10 μg/kg bolus plus 0.15 μg/kg/min infusion for 18–36 hours). The primary end point was net adverse clinical events (NACE) at 30 days defined as a composite of death from any cause, reinfarction, ischemia-driven target vessel revascularization (TVR), stroke, or any bleeding. Secondary end points included NACE at 1 year, major adverse cardiac and cerebrovascular events (MACCE), and any bleeding (Bleeding Academic Research Consortium [BARC] definition) at 30 days and 1 year. Stent thrombosis (ARC definite or probable) at 30 days and 1 year and thrombocytopenia (nadir platelet count < 100 × 109/L or drop > 50% from baseline) at 30 days were the safety end points.
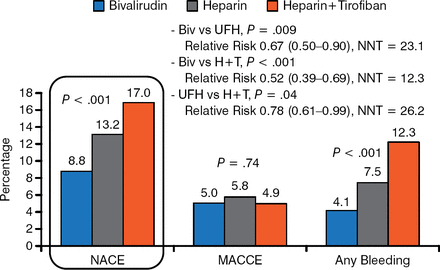
Participants were mean age 58 years, 82% were men. The majority (88%) enrolled with STEMI within 7 hours of first symptom. Mean door-to-device time was 68 minutes. Almost all (99%) patients received drug-eluting stents; most stents (78%) were placed using transradial access. At 30 days, NACE (P < .001) and bleeding (P < .001) occurred significantly less often in bivalirudin-treated patients compared with the heparin therapies (Figure 1). Time-to NACE, but not MACCE, favored bivalirudin (P < .001) at 30 days.
NACE, MACCE, and Any Bleeding at 30 Days
Biv, bivalirudin; H + T, heparin plus tirofiban; MACCE, major adverse cardiac and cerebrovascular events; NACE, adverse clinical events; UFH, unfractionated heparin.
Reproduced with permission from Y Han, MD.
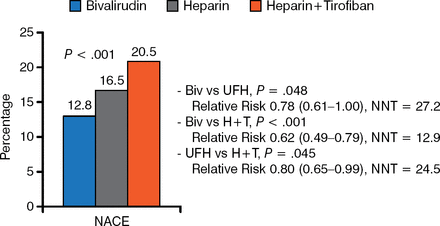
Thrombocytopenia occurred in 0.1%, 0.7%, and 1.1% of bivalirudin-, heparin-, and heparin plus tirofiban-treated patients, respectively (P = .02, bivalirudin vs heparin plus tirofiban). No group differences for ischemic events or stent thrombosis (STEMI) were noted at 30 days. At 1 year, NACE and time-to-NACE also favored bivalirudin (Figure 2).
NACE at 1 Year
Biv, bivalirudin; H + T, heparin plus tirofiban; NACE, adverse clinical events; UFH, unfractionated heparin.
Reproduced with permission from Y Han, MD.
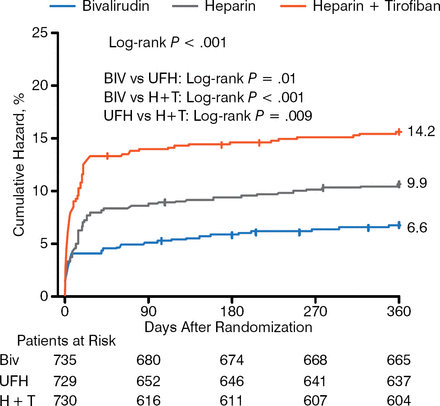
There were no significant differences as to major ischemic events at 1 year; however, bleeding risk favored bivalirudin (Figure 3).
Treatment-Related Bleeding
Biv, bivalirudin; H + T, heparin plus tirofiban; UFH, unfractionated heparin.
Reproduced with permission from Y Han, MD.
The open-label trial design limits interpretation of these findings, as does the unequal number of STEMI and NSTEMI participants. In addition, Chinese domestic bivalirudin was used in this study. Prasugrel and ticagrelor were not used, as they were not available in China at the time of enrollment.
- © 2014 MD Conference Express®