Summary
Throughout the past year, results from multiple clinical trials in the field of cardiology have been released. This article reviews data that were released from the top 10 clinical trials in cardiovascular health during the past year.
- Cardiology Clinical Trials
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
Throughout the past year, results from multiple clinical trials in the field of cardiology have been released. Gregory R. Giugliano, MD, SM, Baystate Medical Center, Springfield, Massachusetts, USA, reviewed data that were released from the top 10 clinical trials in cardiovascular health during the past year.
In the continued search for an oral anticoagulant (OAC) that can be used in patients with mechanical heart valves at risk of developing stroke, the Phase 2, dose validation RE-ALIGN trial evaluated dabigatran, a novel OAC (NOAC), compared with warfarin in patients who had received a mechanical aortic- or mitral-valve replacement within the previous 7 days or 3 months [Eikelboom JW et al. N Engl J Med 2013]. All doses of dabigatran (150 mg, 220 mg, or 300 mg BID of dabigatran depending on kidney function) were inferior to warfarin in reducing stroke and bleeding, because thrombosis, primary strokes, and bleeding rates were increased in the dabigatran arm compared with the warfarin arm, resulting in early discontinuation of the trial by the US Food and Drug Administration (FDA). Therefore, warfarin remains the mainstay of treatment for patients with mechanical heart valves who require stroke prophylaxis.
In the prevention of recurrent symptomatic venous thromboembolism (VTE), the Phase 3 Hokusai-VTE trial was designed to determine if the NOAC edoxaban, a factor Xa inhibitor, was noninferior to warfarin [Büller HR et al. N Engl J Med 2013]. Edoxaban was demonstrated to be noninferior to warfarin, because patients in the edoxaban arm experienced significantly fewer recurrent VTE compared with patients in the warfarin arm (HR, .89; 95% CI, .70 to 1.13; p < .001 for noninferiority), including a subgroup with right ventricular dysfunction (HR, .52; 95% CI, .28 to .98). In addition, the rate of major or clinically relevant nonmajor bleeding was lower with edoxaban treatment compared with warfarin (HR, .81; 95% CI, .71 to .94; p = .004 for superiority).
Edoxaban was compared with warfarin in patients with nonvalvular atrial fibrillation (AF) at risk of developing stroke in the ENGAGE AF-TIMI 48 trial [Giugliano RP et al. N Engl J Med 2013]. Similar to the other NOAC trials, high-dose edoxaban (60 mg) was demonstrated to be noninferior to warfarin in the reduction of stroke and systemic embolism (HR, .79; 95% CI, .63 to .99; p < .001 for noninferiority) with lower rates of major bleeding (HR, .80; 95% CI, .71 to .91; p < .001).
Dr. Giugliano highlighted that there are currently 3 NOACs available to clinicians, dabigatran, rivaroxaban, and apixaban, with edoxaban awaiting FDA approval. In the large randomized trials that evaluated the NOACs, one consistent theme emerged because they all appear to reduce the rate of intracranial hemorrhage compared to warfarin. In patients with acute coronary syndrome (ACS) of unstable angina (UA) or non-ST elevation myocardial infarction (NSTEMI), the randomized Phase 3 ACCOAST trial evaluated the effect of pretreatment with prasugrel or prasugrel administered at the time of πercutaneous coronary intervention (PCI) [Montalescot G et al. N Engl J Med 2013]. Unexpectedly, prasugrel pretreatment did not decrease the rate of ischemic events for up to 30 days, and it increased the rate of major bleeding. Clopidogrel and ticagrelor are already FDA approved for this indication, but it appears that there is no benefit for prasugrel pretreatment in this patient population.
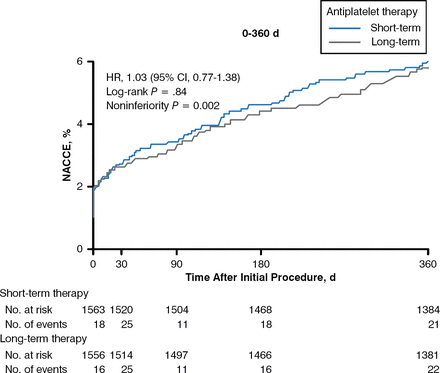
In some cases, antiplatelet therapy must be stopped in patients who have received a drug-eluting stent (DES) to accommodate the need for surgery or as a result of a bleeding episode. The randomized, noninferiority OPTIMIZE trial evaluated 3 months versus 12 months of dual antiplatelet therapy (DAPT) with aspirin plus clopidogrel following placement of a zotarolimus DES in patients with low-risk ACS [Feres F et al. JAMA 2013]. There was no significant difference between the 2 periods and the composite score of all-cause death, MI, and major bleeding (HR, 1.12; 95% CI, .87 to 1.45) at 1 year, as well as similar rates of stent thrombosis (Figure 1). Dr. Giugliano cautioned, however, that there was insufficient statistical power to assess differences in stent thrombosis, which is the chief concern when patients discontinue DAPT early.
Effect of Dual Antiplatelet Therapy Duration After Drug-Eluting Stent Placement
d=days; NACCE=net adverse clinical and cerebral events (composite of all-cause mortality, myocardial infarction, stroke, and major bleeding); No.=number.
Reproduced from Feres F et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013 Dec 18;310(23):2510–2522. With permission from the American Medical Association.
During the past year, 2 major trials were conducted in hypertension involving the kidney. In the CORAL trial, renal artery stenting to resolve renal artery atherosclerotic stenosis in an effort to improve systolic hypertension was compared with medical therapy alone [Cooper CJ et al. N Engl J Med 2014]. Throughout the median follow-up time of 43 months, there was no significant difference between the 2 arms in the composite end point of death from cardiovascular or renal causes, MI, stroke, hospitalization for congestive heart failure, progressive renal insufficiency, or need for renal-replacement therapy (HR, .94; 95% CI, .76 to 1.17; p = .58), as well as individual components of the composite score or all-cause mortality. There was a modest improvement in systolic blood pressure in patients who received the renal artery stent (–2.3 mm Hg; 95% CI, −4.4 to –.2; p = .03). Dr. Giugliano commented that as a result of the negative data from this trial, renal artery stenting will likely now be infrequently performed in the United States.
A “hot” topic during the past year, renal denervation for the treatment of resistant hypertension, was evaluated in the randomized, blinded, sham-controlled, SYMPLICITY HTN-3 trial [Bakris GL et al. J Am Coll Cardiol 2014]. There was no significant difference in 24 h ambulatory systolic blood pressure change between patients who underwent denervation and the sham procedure at 6 months post procedure (–2.0 mm Hg; 95% CI, −5.0 to 1.1; p = .98), as well as no difference in daytime (–1.1 mm Hg; 95% CI, −4.3 to 2.2; p = .52) or nocturnal ambulatory systolic blood pressure (–3.3 mm Hg; 95% CI, −6.7 to .2; p = .06). Dr. Giugliano pointed out that the patients in both arms of the SYMPLICITY HTN-3 trial were taking about 5 antihypertensive medications (eligibility criteria mandated ≥ 3), indicating that these patients had true resistant hypertension. Although safe, renal denervation did not improve hypertension, a result that highlights the importance of blinding and sham procedures, because previous non-blinded, non-sham-controlled studies had demonstrated positive results.
In interventional cardiology, the Medtronic CoreValve was approved for transcatheter aortic valve replacement after a trial demonstrated noninferiority compared with surgical aortic valve replacement in very high-risk patients. In patients with diabetes and triple-vessel coronary disease, the randomized FREEDOM trial demonstrated that coronary artery bypass grafting (CABG) was superior to PCI in the primary composite end point of death from any cause, nonfatal MI, and nonfatal stroke (p = .005). The rate of stroke, however, occurred more frequently in the CABG arm compared with the PCI arm at 5 years (5.2% vs 2.4%; p = .03).
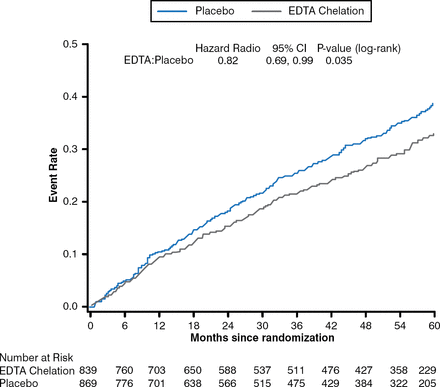
In an interesting approach to treating MI, the TACT trial evaluated the effect of chelation therapy with disodium ethylene diamine tetraacetic acid (EDTA) in patients who had experienced an MI at least 6 weeks prior to enrollment [Lamas GA et al. JAMA 2013]. Patients were randomly assigned to receive a 500 mL infusion of chelation therapy, which included EDTA, ascorbate, B vitamins, electrolytes, procaine, and heparin, or placebo weekly for 30 weeks followed by an additional 10 infusions administered every 2 to 8 weeks; or an oral vitamin and mineral therapy or placebo. Patients who received the chelation therapy experienced a significant decrease in the primary composite end point of total mortality, recurrent MI, stroke, coronary revascularization, or hospitalization for angina compared with patients who received placebo (HR, .82; 95% CI, .69 to .99; p = .035), with similar results for the individual components of the composite score (Figure 2). Dr. Giugliano commented that although the trial has been criticized, he feels that despite the expectation that the results would be negative, the positive results should be respected because of the randomized design of the trial.
Effect of Chelation Therapy in the TACT Trial
EDTA=disodium ethylene diamine tetraacetic acid.
Reproduced from Lamas GA et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309(12):1241–1250. With permission from the American Medical Association.
Finally, Dr. Giugliano presented a randomized, non-blinded study that compared bariatric surgery (gastric bypass or sleeve gastrectomy) plus medical therapy to intensive medical therapy alone in patients with uncontrolled type 2 diabetes mellitus for 12 months of follow-up [Schauer PR et al. N Engl J Med 2012]. Bariatric surgery resulted in a greater improvement in glycemic control as measured by glycated hemoglobin, weight loss, and metabolic syndrome, as well as the use of drugs to lower glucose, lipid, and blood pressure levels. Dr. Giugliano pointed out that more patients who underwent bariatric surgery were able to achieve glycemic control without medical therapy.
In conclusion, positive and negative data have been published for multiple clinical trials during the past year that have the potential to improve outcomes in cardiovascular care. In several cases, new agents failed to improve outcomes, such as prasugrel pretreatment in PCI and dabigatran in patients with mechanical valves. Others showed promise, however, such as edoxaban in nonvalvular AF and the addition of a new percutaneous aortic valve.
- © 2014 MD Conference Express®