Summary
This article discusses the results from the Assessment of Weekly Administration of LY2189265 in Diabetes-6 [AWARD-6; NCT01624259] trial, which show that once-weekly dulaglutide provides glycemic control that is noninferior to once-daily liraglutide with a similar safety and tolerability profile.
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
Results from the Assessment of Weekly Administration of LY2189265 in Diabetes-6 [AWARD-6; NCT01624259] trial, presented by Santiago Tofé Provedano, MD, Clinica Juaneda, Endocrinologia, Palma de Mallorca, Spain, show that once-weekly dulaglutide provides glycemic control that is noninferior to once-daily liraglutide with a similar safety and tolerability profile [Dungan KM et al. Lancet. 2014].
AWARD-6 was a phase 3 randomized, open-label, parallel-arm, 26-week study comparing the efficacy and safety of once-weekly dulaglutide 1.5 mg (n = 299), a long-acting glucagon-like peptide–1 receptor agonist, with once-daily liraglutide 1.8 mg (n = 300). Liraglutide was initiated at a dose of 0.6 mg/d and was titrated to 1.2 mg/d in week 2 and 1.8 mg/d in week 3. The study comprised type 2 diabetes (T2D) patients with an HbA1c ≥ 7% and ≤ 10% who were on a stable dose of metformin (≥ 1500 mg) for ≥ 3 months.
Participants (∼ 50% women and mostly white [86%]) had a mean age of 57 years and a mean HbA1c of 8.1%. Most were obese (mean body mass index [BMI] 34 kg/m2). The mean duration of diabetes was 7 years; mean daily metformin dose was more than 2000 mg. The primary study endpoint was the noninferiority of the change in HbA1c from baseline to 26 weeks using a noninferiority margin of 0.4%. Superiority at week 26 (controlled for Type 1 error) was a key secondary endpoint and was to be tested if the noninferiority endpoint was met.
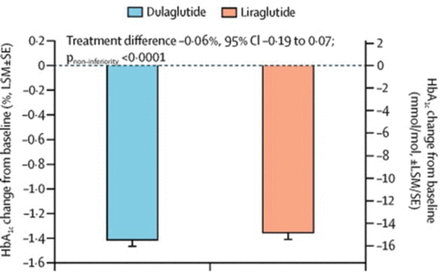
Dulaglutide was noninferior to liraglutide at 26 weeks (mean difference in HbA1c −0.06%; 95% CI, −0.19 to 0.07; P noninferiority < .001; Figure 1). The reductions in HbA1c were similar throughout time and did not differ based on HbA1c at baseline (ie, < 7.0% vs ≤ 6.5%). The effect on postprandial and fasting blood glucose was similar with both drugs. The mean difference in HbA1c was not superior with dulaglutide as compared with liraglutide.
HbA1c Change From Baseline at Week 26
LSM, least squares mean.
aTreatment difference (nominal 95%) CI, mixed-model repeated-measures analysis.
Reprinted from The Lancet, 384, Dungan KM, Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial, 1349–1357, Copyright 2014, with permission from Elsevier.
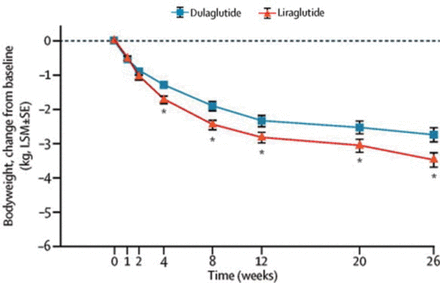
Both groups experienced significant weight reduction; however, patients treated with liraglutide had greater reductions in weight compared with dulaglutide-treated patients (0.7 kg, P < .05, Figure 2).
Body Weight Change Throughout Time
LSM, least squares mean.
* P < .05.
Reprinted from The Lancet, 384, Dungan KM, Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial, 1349–1357, Copyright 2014, with permission from Elsevier.
Similar numbers of patients reported adverse events (AEs). As expected, the most common AEs were gastrointestinal. These included nausea (20% and 18% for dulaglutide and liraglutide, respectively), diarrhea (both 12.0%), dyspepsia (8% and 6%), and vomiting (7% and 8%). The rate of study or study drug discontinuation due to any AE was similar (18 [6%] in each group). The rate of hypoglycemia (≤ 3.9 mmol/L ± symptoms) events was low: 0.3/patient/y with dulaglutide and 0.5 with liraglutide. No episodes of severe hypoglycemia were reported. There were no incidences of adjudicated pancreatitis or pancreatic cancer.
The once-weekly dosing regimen of dulaglutide allowed patients to administer substantially fewer injections while achieving similar glycemic benefits. In this study, the compliance rate (defined as patients achieving > 75% of prescribed doses) for dulaglutide was 98.5% compared with 97.8% for liraglutide [Dungan KM et al. Lancet. 2014]. An additional study is needed to determine whether long-term use of once-weekly drugs like dulaglutide might improve treatment compliance compared with more frequently administered regimens.
- © 2014 MD Conference Express®