Summary
Cell-replacement strategies to preserve insulin production and closed-loop continuous glucose monitoring and insulin delivery systems are attractive options for exogenous insulin delivered through several daily injections for patients with type 1 diabetes (T1D). This article discusses advances in cellular-based therapies for T1D, update of closed-loop therapy, the cost of research efforts to find a cure, as well as reviews attempts to prevent the development of T1D.
- Prevention & Screening Insulin
- Diabetes Mellitus
- Prevention & Screening
- Endocrinology
- Diabetes & Metabolic Syndrome
- Insulin
- Diabetes Mellitus
Cell-replacement strategies to preserve insulin production and closed-loop continuous glucose monitoring and insulin delivery systems are attractive options for exogenous insulin delivered through several daily injections for patients with type 1 diabetes (T1D).
Todd M. Brusko, PhD, University of Florida, Gainesville, Florida, USA, provided an update on advances in cellular-based therapies for T1D, noting that regulatory T cells (Tregs) are central in controlling T-cell activation and preventing the development of T1D, and could represent a new therapeutic paradigm because of the diversity of their functional capacity. Tregs have the ability not only to block autoreactive effector T cells, the cells that elicit β cell destruction, but also to limit inflammation. Therefore, harnessing Tregs and directing them to sites of inflammation could specifically target autoimmune disease.
The function of Tregs in patients with T1D is not defective, he said. Rather, a subset of patients has defects in the suppressor function of Tregs, thereby influencing the pathogenesis of T1D. Autologous umbilical cord blood, which contains a robust population of “naïve” highly functional Tregs, is being explored as a source of these immunomodulatory cells for the treatment of autoimmune diseases including T1D. A trial involving infusing Tregs into adults with T1D is underway led by Jeffrey Bluestone, PhD, University of California, San Francisco, San Francisco, California, USA. Efforts are currently underway to developing additional Treg therapies from umbilical cord blood.
Antigen-specific Tregs have been shown to prevent and even reverse diabetes in animal models. Efforts to generate antigen-specific human Tregs to suppress the immune response targeting islet β cells, while leaving intact the immune response to foreign agents, are ongoing, said Dr. Brusko. In addition, his laboratory is developing a “negative vaccine” in an attempt to block the immune response directed at insulin that is present in patients with T1D. The vaccine uses time-released biomaterials loaded with autoantigens to direct T-cell responses to one of immunologic tolerance and nonresponsiveness to specific proteins such as insulin.
PREVENTION EFFORTS
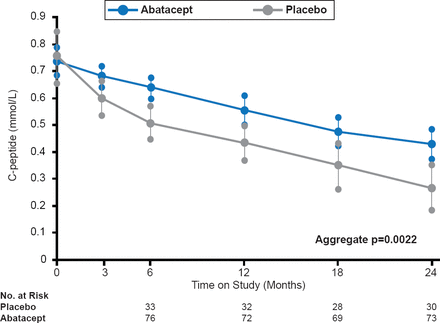
Srinath Sanda, MD, University of California, San Francisco, San Francisco, California, USA, reviewed attempts to prevent the development of T1D. The decision to use drugs in prevention is not yet informed by a mechanistic understanding of why the drugs have been successful in the new-onset population, she said. In one study, modulation of costimulatory signals between T cell and antigen-presenting cells using abatacept slowed the decline of β cell function over 2 years in a study of patients with recent-onset T1D (Figure 1) [Orban T et al. Lancet 2011]. The ideal time to apply immunotherapeutic agents to potentially prevent T1D remains unknown.
Decline in β Cell Function Over Time According to C-Peptide Levels
Reproduced from Orban T et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378(9789):412–419. With permission from Elsevier.
The role of innate inflammation in new-onset T1D is evident by elevations in expression of interleukin (IL) 1B by immune effector cells, leading to hyperglycemia-induced β cell dysfunction. Antagonists of IL-1 (canakinumab and anakinra), however, did not improve β cell function in T1D of recent onset in randomized, double-blind, placebo-controlled trials [Moran A et al. Lancet 2013]. Other data indicate that signaling through receptors that use the MyD88 adaptor protein is critical for development of T1D, and deficiency of MyD88 changes the composition of distal gut microbiota to that interaction between intestinal microbes and the innate immune system is critical to a predisposition to T1D [Wen L et al. Nature 2008]. Factors that influence β cell function before a clinical diagnosis of T1D, the relevant perturbations in the immune system prior to clinical diagnosis, the timing of their occurrence in relation to disease progression, and the mechanisms by which therapies induce a clinical response in the new-onset period require further study, said Dr. Sanda.
CLOSED-LOOP ALGORITHMS
Andrew A. Bremer, MD, PhD, Vanderbilt University, Nashville, Tennessee, USA, provided an update of closed-loop therapy. The goal of an artificial pancreas is the coupling of a continuous glucose monitor and insulin pump therapy with a control algorithm to deliver insulin in a continuous glucose-responsive manner.
Closed-loop control algorithms respond with insulin infusion that reacts to current glucose, accounts for glucose level over time, and can adjust for the rate of change in glucose. Insulin delivery algorithms based on β cell physiology use an insulin feedback feature that reduces insulin delivery in a manner analogous to the “insulin-onboard” feature of the insulin pump [Palerm CC. Comput Methods Programs Biomed 2011]. Modified algorithms have been able to control hyperglycemia and reduce late post-meal hypoglycemia even with the additional challenge of daytime exercise [Steil GM et al. J Clin Endocrinol Metab 2011].
Model predictive control (MPC) is an algorithm that seeks to optimize blood glucose over time based on models that take into account previous continuous blood glucose measurements and insulin infusion rates, and uses these data to predict future glucose and insulin infusion requirements. The model is then updated based on error in prediction, thereby minimizing the difference between the blood glucose level the model predicts and the target blood glucose.
A bihormonal closed-loop system using insulin and glucagon in separate pumps under individual control algorithms (MPC-based for insulin, proportional-integral-derivative-based for glucagon) achieved a mean blood glucose concentration of 140 mg/dL and prevented hypoglycemia while achieving a mean blood glucose concentration of 164 mg/dL in separate experiments in patients with T1D [El-Khatib FH et al. Sci Transl Med 2010].
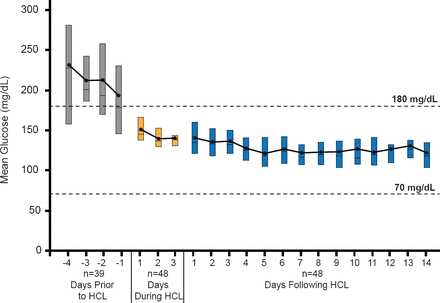
An inpatient hybrid closed-loop therapy initiated within 7 days of a diagnosis of T1D rapidly reversed glucose toxicity and established near-normal glycemic control, with mean glucose concentration falling from 240 mg/dL on initiation to 146 mg/dL on Day 1, a level of control that was sustained on Days 2 and 3 (Figure 2) [DirecNet Study Group. Diabetes Technol Ther 2013].
Effects of Inpatient Hybrid Closed-Loop Therapy Started Within 7 Days of Type 1 Diabetes Diagnosis
Reproduced from DirecNet Study Group. The Effects of Inpatient Hybrid Closed-Loop Therapy Initiated Within 1 Week of Type 1 Diabetes Diagnosis Diabetes Research in Children Network (DirecNet) and Type 1 Diabetes TrialNet Study Groups*. Diabetes Technol Ther 2013;15(5):401–408. With permission from Mary Ann/Liebert, Inc. Publishers.
Wireless wearable ambulatory closed-loop systems that integrate continuous glucose monitoring, continuous subcutaneous insulin infusion, and a controller algorithm onto a Smartphone device are under investigation. Sandra Puczynski, PhD, Southern Illinois University, Springfield, Illinois, USA, discussed patient hopes for a cure for T1D in the face of the reality of research efforts in this endeavor. At least $1.8 billion annually, diabetes research expenditures are not keeping up with the costs associated with diabetes, she said. The status of research is often misrepresented in the media, with discoveries sometimes portrayed as being further along in clinical development than they actually are, causing premature excitement among patients.
- © 2013 MD Conference Express®