Summary
This article discusses data from the ASSERT trial that was designed to test whether treatment with RVX-208, an oral drug that induces apolipoprotein A1 (apoA1) synthesis, would lead to increased apoA1 levels.
- Cardiology Clinical Trials
- Lipid Disorders
- Coronary Artery Disease
Reducing adverse cardiovascular events through improving reverse cholesterol transport has become an active area of research. While raising high-density lipoprotein cholesterol (HDL-C) remains a major focus, increasing the synthesis of apolipoprotein A1 (apoA1), the primary cholesterol transport protein that is associated with HDL-C, has been suggested as an alternative approach. Stephen J. Nicholls, MD, PhD, Cleveland Clinic, Cleveland, Ohio, USA, presented data from a Phase 2 study that was designed to test whether treatment with RVX-208, an oral drug that induces apoA1 synthesis, would lead to increased apoA1 levels. While an increase in levels of apoA1 was observed across the dosing range of RVX-208, treatment with RVX-208 at individual doses did not significantly increase apoA1 levels compared with placebo.
The primary objectives of the ApoA1 Synthesis Stimulation Evaluation in Patients Requiring Treatment for Coronary Artery Disease (ASSERT; NCT01058018) study were to evaluate the efficacy, tolerability, and safety of oral RVX-208 in patients with stable coronary artery disease. This was a double-blind, randomized, controlled phase 2 trial in 299 patients who were receiving stable statin therapy for at least 30 days and treated for 12 weeks with RVX-208 (50, 100, or 150 mg twice daily) or placebo. Patients had a mean age of 65.8 years and were mostly white men and hypertensive; 29.4% were diabetic, and 17.1% smoked. Baseline HDL-C and apoA1 were 44 mg/dL and 141 mg/dL, respectively.
The primary study outcome was the percentage change in apoA1 from baseline to 12 weeks for each treatment arm compared with placebo. Secondary outcomes were comparisons of the dose- and time-response relationships for apoA1, total cholesterol, HDL-C, low-density lipoprotein cholesterol (LDL-C), non-HDL-C, triglycerides, apoB, and LDL and HDL subclasses over 4, 8, and 12 weeks.
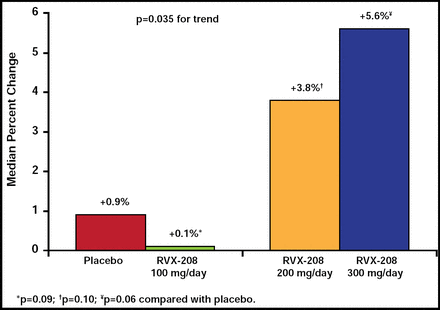
After 12 weeks, there was no statistically significant difference in the increase in apoA1 levels between subjects who were treated with any individual dose of RVX-208 compared with placebo (+5.6% in the 300 mg/day group [p=0.06]; +3.8% in the 200 mg/day group [p=0.10]; and +0.1% in the 100 mg/day group [p=0.09]; Figure 1). However, a dose-dependent increase in apoA1 levels was observed across the dosing range [p=0.035]. RVX-208 produced significantly increased HDL-C levels at the two higher dose levels (+6.3% at the 200-mg/day dose [p<0.05] and +8.3% at 300 mg/day [p<0.01]) but not at the 100-mg/day dose. Although there was no increase in the number of HDL particles, the size of the particles appeared to change, with a preferential increase in the larger HDL particles by up to 21% at the highest dose (p<0.001 versus placebo). There was also a significant (p<0.05) increase in the amount of α1 HDL at the higher doses. There was no change in LDL-C, triglycerides, apoB, or hsCRP.
Median Change in ApoA-1 From Baseline.
Reproduced with permission from S. Nicholls, MD.
Rates of increased ALT/AST levels were higher for each dose of RVX-208 compared with placebo, with 3 patients in the 100 mg/day group, 8 patients in the 200 mg group, and 7 patients in the 300 mg group having ALT/AST >3 xULN (p=0.009). A total of 8 patients had ALT/AST >8 xULN. The liver enzyme increases were reversible, and there was no evidence of liver damage.
“The changes in apoA1, HDL-C, and large HDL particles are consistent with enhanced mobilization of lipids into functional HDL particles and reversible transaminase elevations,” Dr. Nicholls said, thus establishing proof of concept. He also noted that most of the benefits of RVX-208 were seen during Weeks 8 to 12, indicating that longer treatment may have provided an increased benefit. However, the increases in AST/ALT that were seen in this study deserve careful prospective evaluation in future longer-term studies and could limit the clinical utility of this compound.
- © 2010 MD Conference Express