Summary
This article discusses the causes of and options for addressing mitral regurgitation, as well as irregularities in the right ventricular leaflet anatomy, or tricuspid regurgitation, aortic stenosis, surgery for valvular heart disease.
- Heart Failure
- Valvular Disease
- Interventional Techniques & Devices
- Heart Failure
- Valvular Disease
- Cardiology & Cardiovascular Medicine
- Interventional Techniques & Devices
According to Jose Luis Zamorano Gomez, MD, University Hospital, Ramon y Cajal, Madrid, Spain, mitral regurgitation (MR) is a serious problem affecting >10% of Europeans >75 years of age. Dr. Zamorano Gomez then went on to discuss the causes of and options for addressing MR.
MR can result from mitral valve (MV) prolapse or flail leaflets that allow blood to leak back into the left atrium as the heart contracts.
MR should be treated medically and when appropriate can be addressed with repair or replacement. Although some studies have suggested better outcomes with repair relative to replacement, differences in outcome are not clear [Enriquez-Sarno M et al. Circulation 1995]. A thorough assessment of the anatomy, etiology, and quantitative grading of MR is needed to determine the optimal timing and type of surgery. With correct assessment, personalized treatment based on mechanism, grade, and the likely success of MV repair (MVR) is possible. One study showed that patients with asymptomatic MR with effective regurgitant orifice (ERO) sizes ≥40 mm2 were at greater risk for adverse cardiac events compared with those with smaller ERO sizes, and therefore, such patients should be considered for immediate surgery [Enriquez-Sarano M et al. N Engl J Med 2005].
Watchful waiting with appropriate medical therapy has been recommended for patients with asymptomatic MR until they meet criteria for surgery. Patients can be safely followed until the new onset of atrial fibrillation (AF) occurs or recommended anatomic cutoff values are reached (ie, left ventricular [LV] end-systolic diameter ≥45 mm, ejection fraction [EF] <60%, or systolic pulmonary artery pressure >50 mm Hg). With this approach, overall 8-year survival rates of 91% have been reported [Rosenhek R et al. Circulation 2006].
Echocardiographic assessment of valve anatomy and function, as well as the consequences of valvular disease on the cardiac chambers, is an essential part of MR workup [Lancellotti P et al. Eur Heart J Cardiovasc Imaging 2013]. The goal is to delay surgery until the potential benefits justify the risks of intervention but early enough to prevent adverse cardiac events.
George Athanassopoulos, MD, Onassis Cardiac Surgery Center, Athens, Greece, moved the discussion to another valvular regurgitation issue, irregularities in the right ventricular (RV) leaflet anatomy, or tricuspid regurgitation (TR). There are several causes of TR, including functional causes such as pulmonary hypertension (PH), RV dysfunction, AF, and cardiac tumors; structural reasons such as prolapse, congenital anomalies, endocarditis, carcinoid disease, and traumatic injury to the chest; or iatrogenic causes such as pacemaker or defibrillator lead interference, RV biopsy, drugs, and radiation.
Severe isolated TR can also develop late after left-sided valve surgery without left-sided heart failure (HF), PH, or a rheumatic tricuspid valve. The presence of preoperative AF and the preoperative EF are independent determinants of the development of severe isolated TR, while annular dilatation is the main cause [Izumi C et al. Circ J 2011]. There is a significant (p≤0.05) increase in TR with both increased mean pulmonary pressure and annular dilation >1.4 times normal [Casa LD et al. Ann Biomed Eng 2013].
Functional TR can be either idiopathic or PH related. Idiopathic TR is relatively common, associated with aging and AF, excess annular and RV basal enlargement that exhausts valvular/annular coverage reserve, and RV conical deformation that does not cause notable valvular tenting [Topilsky Y et al. Circ Cardiovasc Imaging 2012]. Conversely, TR related to PH is determined by valvular tethering with tenting linked to RV elongation and elliptical or spherical deformation but is unrelated to TR severity.
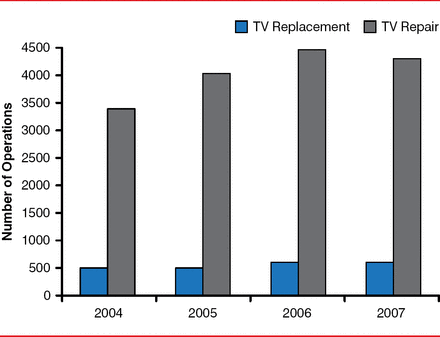
Independent risk factors for progression of TR may include increases in pulmonary artery systolic pressure, the presence of permanent AF, and coronary artery disease. All-cause mortality at 3 years is 20% for patients without TR progression, 42% for those with moderate TR, and 63% for those with severe TR, with increasing severity predicting mortality [Shiran A et al. Am J Cardiol 2014]. Tricuspid annular plane systolic excursion (<15 mm), tricuspid annular systolic velocity (<11 cm/s), and RV end-systolic area (>20 cm2) are used to identify patients with tricuspid value dysfunction. Tricuspid valve repair is performed more often than replacement is (Figure 1) [Roger JH, Bolling ST. Circulation 2009].
More Tricuspid Repairs Performed Compared With Replacements
TV=tricuspid valve.
Reproduced with permission from Lippincott Williams & Wilkins from Roger JH, Bolling ST. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation 2009;119:2718–2725.
Lead-induced TR from RV pacing due to leaflet perforation, entanglement of TV apparatus, and adhesion of an implantable cardioverter-defibrillator or a pacemaker lead to the valve leaflet is associated with long-term complications [Abu Sham'a R et al. Europace 2013]. TR is also common in patients who have undergone heart transplantation and may be related to the biatrial anastomosis technique and graft vasculopathy as well as to reasons for TR in nontransplantation patients [Berger Y et al. J Transplant 2012]. TR is associated with increased mortality in patients with mild to moderate HF but is not an independent predictor of mortality in patients with advanced disease when adjusted for other established risk factors [Neuhold S et al. Eur Heart J 2012].
Although TR and associated complications have been known for many years, the understanding of the underlying etiologies and pathophysiology continues to evolve. The decision of when and how to intervene depends on an increasingly complex network of clinical, anatomic, and surgical data, as well as functional evaluation of the right ventricle.
Aortic stenosis (AS) is the most common heart valve disease, with a prevalence of 4% in adults. In a European heart survey of 1269 patients who underwent intervention for valvular heart disease, prosthetic replacement was performed in 99.0% of patients with aortic disease, percutaneous dilatation in 33.9% of patients with mitral stenosis, and valve repair in 46.5% of patients with MR [Lung B et al. Eur Heart J 2003]. When addressing the issue of aortic valve disease (stenosis and regurgitation), the primary target should be to alleviate symptoms, prevent adverse outcomes, and maintain LV contractility, remarked Konstantinos Toutouzas, MD, Athens Medical School, Athens, Greece.
AS is defined according to flow (normal or low [classical and paradoxical]), gradient (high or low), and EF (preserved or low). One of the most difficult to diagnose and treat valvular heart diseases is low-flow, low-gradient AS, which may occur with preserved or depressed LV EF.
Transcatheter aortic valve replacement (TAVR) is recommended for patients who require surgical valve replacement but who are at high risk for adverse outcomes as a result of the surgery. TAVR can be considered in symptomatic patients with low-flow, low-gradient (<40 mm Hg) AS. Available devices include the Edwards SAPIEN XT (Edwards Lifesciences) and the Medtronic CoreValve (Medtronic).
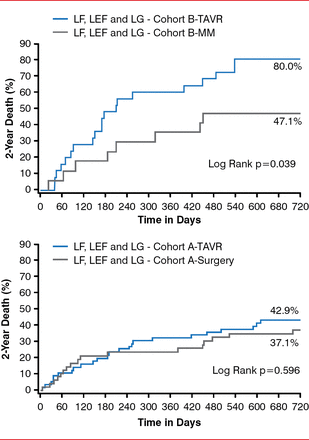
The Placement of Aortic Transcatheter Valves trial studied the safety and effectiveness of transfemoral and transapical device systems compared with medical management in high-risk, symptomatic patients with severe AS [Hermann HC et al. Circulation 2013]. TAVR significantly improved survival (p=0.039) in patients with low flow, low EFs, and low gradients compared with medical management. TAVR produced similar 2-year mortality outcomes compared with surgical aortic valve replacement. Only flow was an independent predictor of mortality (Figure 2).
Two-Year Survival Following TAVR, SAVR, and MM for High-Risk Patients With AS
AS=aortic stenosis; LEF=low ejection fraction; LF=low flow; LG=low gradient; MM=medical management; SAVR=surgical aortic valve replacement; TAVR=transcatheter aortic valve replacement.
Reproduced with permission from Lippincott Williams & Wilkins from Hermann HC, Pibarot P, Hueter I, et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation 2013;127:2316–2326.
With the development of TAVR, balloon aortic valvuloplasty (BAV) has resurfaced. Although not a replacement for TAVR, BAV is an acceptable bridge to SAVR and TAVR in high-risk patients not ready for definitive therapy [Eltchaninoff H et al. Am Heart J 2014]. For predicting suitability for TAVR, the European System for Cardiac Operative Risk Evaluation II may be superior to other risk-scoring methods [Stahile BE et al. Cardiology 2013]. However, dedicated risk scores are still needed, as well as new imaging modalities for the classification of patients with AS.
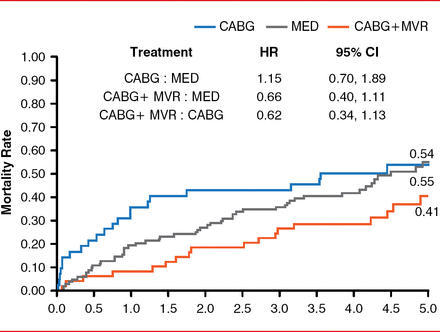
Michele De Bonis, MD, San Raffaele Scientific Institute, Milan, Italy, discussed valvular heart disease from the perspective of a surgeon. There is some evidence that MVR during coronary artery bypass graft surgery (CABG) improves survival in patients with moderate to severe MR compared with CABG alone or just MM (Figure 3) [Deja MA et al. Circulation 2012].
Mortality Rates Following MVR Plus CABG Versus CABG or MM Alone in Patients with Mitral Regurgitation
CABG=coronary artery bypass graft surgery; MED=medical therapy; MM=medical management; MVR=mitral valve repair.
Reproduced with permission from Lippincott Williams & Wilkins from Deja MA, Grayburn PA, Sun B, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation 2012;125(21):2639–2648.
Despite the suggestion of benefit with CABG plus MVR, it is very important to perform a comprehensive assessment of MV configuration and LV geometry and function prior to surgery to assess the presence of predictors of residual or recurrent MR after MVR, because this is related to mortality rate [Magne J et al. Circulation 2007].
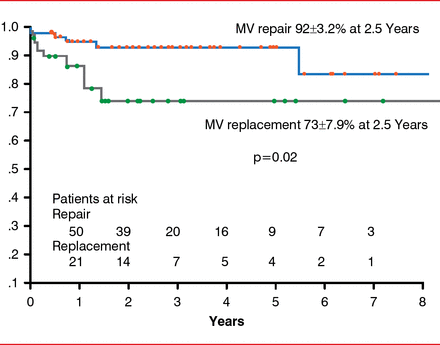
MV repair should be reserved for patients in the early stage of the disease and in the absence of predictors of repair failure, such as high distal mitral anterior leaflet angle, basal mitral posterior leaflet angle > 45°, coaptation depth >1 cm, and too advanced LV remodeling [Lee AP et al. Circulation 2009]. Indeed, recurrence of MR parallels the absence of LV reverse remodeling after repair in advanced dilated cardiomyopathy [De Bonis M et al. Ann Thorac Surg 2008]. A major predictor of reverse LV remodeling is the duration of HF. In patients with ≥1 predictor of repair failure, MV replacement with complete preservation of subvalvular apparatus may be needed. In some series, MV replacement is associated with higher in-hospital and late mortality in patients with advanced dilated and ischemic cardiomyopathy and severe functional MR compared with MVR (Figure 4) [De Bonis M et al. Ann Thorac Surg 2012].
Actuarial Survival Following MV Repair Versus Replacement
MV=mitral valve.
Reproduced with permission from Elsevier from De Bonis M, Ferrara D, Taramasso M, et al. Mitral replacement or repair for functional mitral regurgitation in dilated and ischemic cardiomyopathy: is it really the same? Ann Thorac Surg 2012;94(1):44–51.
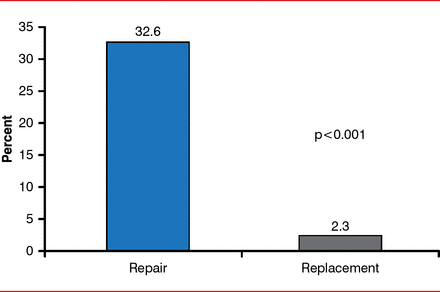
Other studies, however, have shown that for patients with chronic ischemic MR and impaired LV function, MV replacement provides similar survival and higher freedom from reoperation compared with MVR [Lorusso R et al. J Thorac Cardiovasc Surg 2013]. The most recent comparison study reported no significant between-group differences in clinical outcomes between the 2 procedures, although there was significantly more recurrent MR at 1 year with MVR compared with MV replacement (Figure 5) [Acker MA et al. N Engl J Med 2014].
Patients With Recurrent MR at 1 Year Following Repair Versus Replacement
MR=mitral regurgitation.
It must be underlined that in this multicenter study, patients with the previously reported predictors of repair failure were not excluded from the undersized mitral annuloplasty operation, which might explain why the rate of recurrent MR at 1 year was so high. Interestingly, the patients undergoing MVR who had no MR recurrence did show significant LV reverse remodeling. This finding was not observed following MV replacement, suggesting the potential advantage of MVR over MV replacement if the repair is performed in well-selected patients and is therefore successful and durable.
The editors would like to thank the many members of the 2014 Heart Failure presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®