Summary
Patients with type 2 diabetes mellitus (T2DM) appear to have dysfunctional islet β cells due to unknown mechanisms [Leahy JL and Pratley RE. Translational Endo Metab 2011]. Multiple new pharmacologic agents that are under development for the treatment of T2DM specifically target islet cells. This article discusses two signaling pathways in β cells that may be important drug targets, the mechanisms underlying insulin resistance and potential drug targets, information on treating inflammation in adipose and skeletal tissues, as well as targeting mechanisms of glucose absorption and excretion in the treatment of T2DM.
- Exclusive Article - For home page
- Insulin
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Exclusive Article - For home page
- Insulin
- Diabetes Mellitus
Patients with type 2 diabetes mellitus (T2DM) appear to have dysfunctional islet β cells due to unknown mechanisms [Leahy JL and Pratley RE. Translational Endo Metab 2011]. Multiple new pharmacologic agents that are under development for the treatment of T2DM specifically target islet cells. Jack L. Leahy, MD, University of Vermont, Burlington, Vermont, USA, presented two signaling pathways in β cells that may be important drug targets.
Dr. Leahy pointed out that there are several therapeutic targets within β cells for the treatment of T2DM [Bailey CJ. Lancet 2012]. One target is an unsaturated medium- to long-chain free fatty acid receptor called GPR40 (also called FFAR1) that plays a role in free fatty acid-induced insulin secretion [Mancini AD, Poitout V. Trends Endocrinol Metab 2013]. Studies have demonstrated decreased insulin secretion in GPR40 null mice compared with controls [Steneberg P et al. Cell Metab 2005; Kebede M et al. Diabetes 2008]. Interestingly, GPR40 expression in human pancreas islet cells is decreased in patients with T2DM by up to 80%, when compared with controls [Del Guerra S et al. Nutr Metab Cardiovasc Dis 2010].
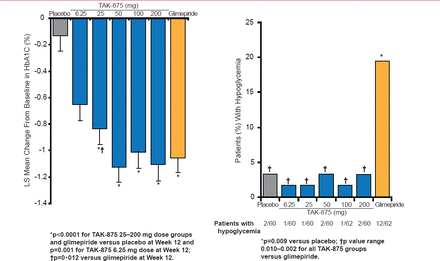
Dr. Leahy highlighted data from a Phase 2 trial in which 426 patients with T2DM were treated with the GPR40 agonist TAK-875, glimepiride, or placebo for 12 weeks [Burant CF et al. Lancet 2012]. Patients were counseled on diet and exercise and may or may not have been stable on metformin. A significant improvement in HbA1C levels was observed in patients who received TAK-875 or glimepiride, compared with those who received placebo (Figure 1). Patients treated with TAK-875 or glimepiride did not have improved insulin sensitivity or fasting glucagon levels. Additionally, treatment-related adverse events were similar among patients who received TAK-875 or placebo. However, a greater number of patients who received glimepiride demonstrated hypoglycemic events, as compared with those who received placebo or TAK-875.
Effects of TAK-875, Glimepiride, and Placebo on Glycemia
Reproduced from Burant CF et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 21012;379(9824):1403–1411. With permission from Elsevier.
Dr. Leahy gave an example of identifying new therapeutic targets in well-studied signaling pathways in β cells, such as the FoxO1 and PPAR-γ pathway in β cells that is important for β cell survival, function, metabolism, and response to incretin. Rats with hyperglycemia demonstrate reduced signaling through the FoxO1/PPAR-γ pathway due to the potentially impaired translocation of FoxO1 from the nucleus to the cytoplasm [Gupta D et al. Diabetes 2010]. Treatment of hyperglycemic rats with the DPP-4 inhibitor alogliptin appears to restore FoxO1/PPAR-γ signaling [Gupta D et al. ADA 2013 (poster 2275)].
Abd A. Tahrani, MD, PhD, University of Birmingham, Edgbaston, United Kingdom, discussed the mechanisms underlying insulin resistance and potential drug targets. Prof. Tahrani pointed out that the most important factor associated with insulin resistance is obesity; however, he noted that it is difficult to treat obesity. Targets for the treatment of insulin resistance include obesity and the mechanisms of insulin resistance such as inflammation, oxidative stress, lipotoxicity, and glucotoxicity, as well as targeting the insulin receptor and insulin post-receptor signaling.
Prof. Tahrani highlighted several drugs that target insulin resistance. The compounds demethylasterriquinone B1, compound 2, and D-410639 target insulin receptor activation [Zhang B et al. Science 1999; Strowski MZ et al. Endocrinology 2004; Tsai KW et al. J Biomed Sci 2009]. Some agents prolong phosphorylation of the β subunit of the insulin receptor following insulin binding [Cohen P et al. Nat Rev Mol Cell Biol 2006], such as the agent TLK16998 [Manchem VP et al. Diabetes 2001]. Other targets include interleukin-6 [Ridker PM et al. Circulation 2012], resveratrol [Ito-Nagahata T et al. Biosci Biotechnol Biochem 2013], and inhibitor κ-B kinase-β inhibitors [Kamon J et al. Biochem Biophys Res Commun 2004].
Postreceptor insulin signaling may also harbor potential targets for the treatment of insulin resistance. Prof. Tahrani highlighted signaling molecules within the insulin signaling pathway that may be ideal targets, such as inhibition of protein kinase C [Naruse K et al. Diabetes 2006], promotion of phosphatidylinositol-3 kinase [Croze ML et al. Biochimie 2013], inositol metabolites, inhibition of PTEN [Pal A et al. N Engl J Med 2012], and inhibition of inositol phosphatases.
Prof. Tahrani pointed out that nonpharmacologic interventions, such as weight loss, adequate sleep, and obstructive sleep apnea are also important to consider. For example, patients who were sleep restricted in a laboratory study had a significant decrease in glucose tolerance, acute insulin response to glucose, glucose effectiveness, and insulin sensitivity as compared with a well-rested state [Leproult R, Van Cauter E. Endocr Dev 2010].
Robert R. Henry, MD, University of California, San Diego, La Jolla, California, USA, presented information on treating inflammation in adipose and skeletal tissues. Individuals that tend to accumulate adipose tissue in the visceral region are more likely to have inflammation in their adipose tissue. In addition, patients with T2DM have greater fat deposition in the visceral versus subcutaneous region, as compared with patients without diabetes.
Dr. Henry highlighted that adipose tissue produces chemicals called adipokines, which can have autocrine, paracrine, or endocrine signaling effects [Blüher M. Diabetes Metab J 2012]. As adiposity increases, the adipokines that are secreted become predominantly proinflammatory. A prevailing theory of a mechanism underlying insulin resistance in obesity is lipotoxicity [Johnson AM, Olefalsy JM. Cell 2013]. Excessive caloric intake can result in cellular stress and tissue inflammation, which can lead to insulin resistance [Odegaard JI, Chawla A. Science 2013]. Ultimately, inflamed adipose tissue with some level of metabolic dysfunction demonstrates an altered milieu as compared with lean adipose tissue with normal metabolic function [Ouchi N et al. Nat Rev Immunol 2011]. Adipocytes that live in an inflamed tissue have reduced insulin action [Odegaard JI, Chawla A et al. Science 2013].
A recent study demonstrated that treatment with salsalate, a tumor necrosis factor-α inhibitor, resulted in a significant decrease in white blood cell count, neutrophil count, and lymphocyte count over a 48-week period [Goldfine AB et al. Diabetalogia 2013]. In addition, salsalate treatment caused a reduction in nuclear factor kappa-light chain enhancer (NF-κB) activity in visceral adipose tissue, compared with placebo, after 12 weeks of treatment. Importantly, NF-κB is a major player in inflammatory signaling pathways [Reilly SM et al. Nat Med 2013].
Inflammation of skeletal muscle is increased in obese patients compared with lean patients, as measured by macrophage infiltration and, as body mass index increases, so does the macrophage content of skeletal muscle [Varma V et al. Am J Physiol Endocrinol Metab 2009]. However, other studies have suggested that this finding may be due to cross-contamination by adipose tissue that is found within skeletal tissue [Tam CS et al. Obesity 2012].
Bernard Zinman, CM, MD, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, University of Toronto, Toronto, Canada, discussed targeting mechanisms of glucose absorption and excretion in the treatment of T2DM. The alpha-glucosidase inhibitors [AGI] are a drug class that modifies glucose absorption by inhibiting the breakdown of carbohydrates in the upper intestine. Interestingly the AGIs also modify the secretion of gastrointestinal (GI) peptides like GLP1 but have modest efficacy. However, the tolerability of AGI is a major problem and their widespread acceptance in the United States has been limited by frequent GI side effects, such as diarrhea, flatulence, and abdominal distention.
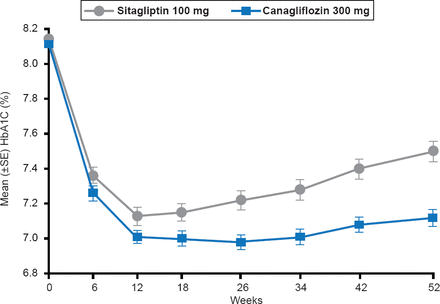
A new strategy focuses on targeting the sodium glucose cotransporter-2 (SGLT-2), which is an important mechanism responsible for the filtered glucose to be reabsorbed in the proximal tuble of the kidney. Nonetheless the capacity of the two transporters, SGLT-1 [high-affinity, low-capacity] and SGLT-2 [low-affinity, high-capacity] to reabsorb glucose is limited; therefore, excess blood glucose levels above ∼180 mg/dL causes glucose to remain in the urine filtrate [Gerich JE et al. Diabetic Med 2010]. However, in patients with poorly controlled diabetes there is an adaptive response whereby SGLT-2 is upregulated so that reabsorption of glucose is increased. Thus SGLT-2 inhibitors reduce glucose reabsorption in the kidney and with resultant glucosuria [increased urinary glucose]. Several SGLT-2 inhibitors that have shown promising results in clinical trials include empagliflozin [Hach T et al. Diabetes 2013], canagliflozin (Figure 2) [Schernthaner G et al. Diabetes Care 2013], and dapagliflozin [FDA. Dapagliflozin Advisory Committee Meeting. http://www.fda.gov/downloads/AdvisoryCommittees/CommiitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM264314.pdf. Published July 19, 2011].
Effect of Canagliflozin Versus Sitagliptin on HbA1C Levels
Reproduced from Schernthaner G et al. Canagliflozin Compared With Sitagliptin for Patients With Type 2 Diabetes Who Do Not Have Adequate Glycemic Control With Metformin Plus Sulfonylurea: A 52-week randomized trial. Diabetes Care 2013;36(4):908–913. With permission from the American Diabetes Association.
- © 2013 MD Conference Express®