Summary
In critically ill patients, the CALORIES trial showed no significant difference between 2 feeding methods on all-cause mortality; the MetaPlus trial results suggest no benefit from administration of an immune-enhanced enteral formula; in the TARGET trial, a higher-density enteral formula led to higher daily caloric intakes; and results from the ICU Microbiome Project indicate that loss of microbiome diversity may lead to poor clinical outcomes.
- critical care
- ICU
- enteral
- parenteral
- nutrition
- microbiome
Four speakers summarized late-breaking results from 4 clinical nutrition trials in critically ill patients and discussed the application of their results to bedside practice.
CALORIES Trial
According to data presented by Richard Beale, MB, BS, King’s College, London, United Kingdom, early parenteral nutrition is neither more harmful nor more beneficial than nutrition delivered via the enteral route.
There is ongoing debate whether enteral or parenteral nutrition is the optimal nutrition support for critically ill patients [Simpson F, Doig G. Intensive Care Med. 2005; Gramlich L et al. Nutrition. 2004; Heyland DK et al. JPEN J Parenter Enteral Nutr. 2003]. Prof Beale presented results from the recently published CALORIES trial [Harvey S et al. N Engl J Med. 2014], a real-world study designed to examine whether parenteral feeding was superior to enteral feeding in patients in the intensive care unit (ICU).
CALORIES was a randomized controlled trial (RCT) that included > 2300 hospitalized adults who experienced an unplanned admission to 1 of 33 ICUs in England. Eligible patients were expected to require nutrition support for ≥ 2 days during an ICU stay of ≥ 3 days. Patients were randomly assigned to either parenteral or enteral feeding within 36 hours of admission, with nutrition support continued for up to 5 days. The primary outcome measure was all-cause mortality at 30 days. The study had a number of secondary outcomes that included infectious and noninfectious complications, length of ICU and hospital stay, and duration of survival up to 1 year.
Data were collected from 2388 patients in the parenteral (n = 1191) and enteral (n = 1197) groups. At 30 days, there was no significant difference in the primary outcome measure between the 2 groups (P = .57). Patients in the parenteral group were significantly less likely to experience hypoglycemia (P = .006) or vomiting (P < .001). No significant differences were noted in other secondary outcomes. The majority of patients in both groups received less than the predetermined target delivery of 25 kcal/kg/d.
There was no increase in infectious complications as reported by previous studies, perhaps due to better managing vascular access, to preventing ventilator-assisted pneumonia, or to improvements in feeding technology. Prof Beale offered several hypotheses regarding why the caloric targets were not met. While this was expected in the enteral group, there may have been content and delivery issues that delayed or interrupted scheduled feedings in the parenteral group.
MetaPlus Trial
Data suggest that immune-modulating nutrients such as glutamine, omega-3 fatty acids, selenium, and antioxidants can reduce infections and improve recovery from critical illness. However, there is a lack of consensus among professional organizations regarding the benefit of administering enteral nutrition products that contain these nutrients (Table 1).
Enteral Nutrition Guidelines for Critically Ill Patients
Arthur Van Zanten, MD, PhD, Gelderse Vallei Hospital, Ede, the Netherlands, described results from the MetaPlus trial [Van Zanten A et al. JAMA. 2014], a double-blind multicenter RCT conducted in 14 European ICUs. The study ran for 26 months, including a 6-month follow-up. The goal of MetaPlus was to evaluate whether the incidence of infection in mechanically ventilated patients in the ICU could be reduced by administering high-protein enteral nutrition enriched with immune-modulating nutrients (IMHP). The control group received standard high-protein enteral nutrition (HP).
A total of 301 adults were randomized to receive IMHP (n = 152) or HP (n = 149). Data were analyzed on an intention-to-treat basis that included all patients. Data from prespecified medical, surgical, and trauma subgroups were also analyzed. The primary outcome measure was incidence of new infections; secondary outcomes included the effect of each formula on mortality, Sequential Organ Failure Assessment scores, the duration of mechanical ventilation, and the length of ICU and hospital stay.
There was no significant difference between the IMHP and HP groups relative to the primary outcome in either the entire population or the subpopulations (Table 2). No statistically significant differences were observed in other end points, except for a higher 6-month mortality rate in the medical subgroup (P = .04). Prof Van Zanten concluded that the MetaPlus results do not support the use of IMHP for adult patients in the ICU on mechanical ventilation. There is no benefit and some concern about increased mortality in the subgroup of medical patients.
Incidence of New Infections by Enteral Nutrition Product
The ICU Microbiome Project
Paul Wischmeyer, MD, University of Colorado School of Medicine, Aurora, Colorado, USA, posed the question of whether the gut bacteria that physicians try to eradicate in patients might be highly beneficial, and he presented data suggesting that patients might be better served if these bacteria were replenished rather than destroyed.
Antibiotics and other clinical factors in the ICU can wreak havoc on the gut microbiome. Inflammatory changes, physiologic stress, and antibiotics can incite a cytokine storm, which stimulates the production of nosocomial pathogens [Alverdy JC, Chang EB. J Leukoc Biol. 2008]. Other research suggests that alterations in gut flora are associated with septic complications and even death in patients with a condition known as systemic inflammatory response syndrome [Shimizu K et al. Dig Dis Sci. 2011].
A number of studies and meta-analyses suggest that probiotics may restore balance to the gut flora and reduce the incidence of antibiotic-associated diarrhea [Goldenberg JZ et al. Cochrane Database Syst Rev. 2013; Hempel S et al. JAMA. 2012] and ventilator-assisted pneumonia [Morrow LE et al. Am J Respir Crit Care Med. 2010].
Dr Wischmeyer highlighted the ICU Microbiome Project, which is ongoing in 4 sites throughout the United States and Canada. The objective of the study is to define changes that occur in the microbiomes of patients in the ICU, what effect these changes have on patient outcomes, and how nutrition interventions affect the microbiome in these patients.
Ventilated adult patients in the ICU who were taking antibiotics were assessed at sites participating in the International Critical Care Nutrition Survey. At baseline and day 10 (or at the ICU discharge date), swabs were taken from the patients’ stool, mouth, and skin to create a microbiome bar code that might predict which patients are at high risk of mortality or infection, as well as those who might benefit from specific probiotics or a stool transplant. The data from 149 patients were compared with those from healthy participants in the American Gut (AG) Project.
Overall, there was a clear shift to more pathogens and fewer healthy flora. Compared with the AG cohort, critical illness led to an overgrowth of Bacteroidetes, Staphylococcus aureus, and Enterobacteriaceae and a decrease of Firmicutes and Faecalibacterium prausnitzii.
The hypothesis of the project was that loss of diversity in the gut microbiome would be associated with poorer outcomes. According to Dr Wischmeyer, early data suggest an association between death and an excess of Klebsiella and Enterobacteriaceae on the skin. Bacteremia was associated with Mycoplasma in the mouth. Increased length of stay was associated with an abundance of fecal Bilophila. Better protein nutrition was significantly associated with an increased abundance of Streptococcus anginosus (P < .05). Greater diversity of the biome was associated with a shorter length of stay.
Dr Wischmeyer emphasized that loss of diversity in the microbiome is likely to lead to poor clinical outcomes, and the continuing analysis of these data may provide some guidance toward how to best restore microbial balance in critically ill patients in the ICU.
The TARGET Trial
While the optimal calorie goal for critically ill patients is unclear, most guidelines suggest a goal of 25 kcal/kg/d; however, enteral feedings may deliver only half of that goal. Adam Deane, MD, PhD, University of Adelaide, Adelaide, South Australia, on behalf of the TARGET study investigators and the Australian New Zealand Intensive Care Society, discussed the TARGET trial [Peake S et al. Am J Clin Nutr. 2014], a feasibility study designed to test whether an energy-dense caloric enteric feeding solution would provide more calories than a standard formulation when delivered at the same rate to critically ill patients. The secondary goal was to determine if these data would inform the feasibility of conducting a multicenter double-blind randomized trial.
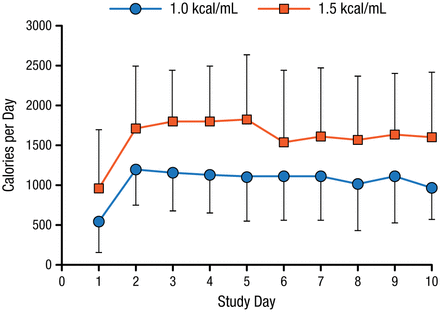
There were 112 patients from 5 Australian ICUs enrolled in TARGET. The mean age was 56 years, and 74% were men. All patients were mechanically ventilated and were expected to require enteral nutrition for ≥ 2 days. Patients were randomized to receive an enteral solution of 1.5 kcal/mL (n = 57) or 1.0 kcal/mL (n = 55) at a rate of 1 mL/kg of ideal body weight per hour for up to 10 days. The primary outcome measure was the number of calories delivered from enteral nutrition per day.
The 2 groups received similar mean volumes of enteral nutrition solution. The higher calorie solution was associated with a significantly higher daily caloric intake (1832 ± 381) when compared with the standard formula (1259 ± 428; P < .001; Figure 1). The higher energy-dense feeds were not associated with larger gastric residual volumes or diarrhea. There were fewer deaths among the patients who received the higher calorie solution 90 days after enrolling in the study, but this was not statistically significant.
Mean Daily Calories Provided by 2 Enteral Solutions
Peake S et al. Am J Clin Nutr. 2014.
Adapted from Peake SL et al. Use of a concentrated enteral nutrition solution to increase calorie delivery to critically ill patients: a randomized, double-blind, clinical trial. Am J Clin Nutr. 2014;100:616-625. With permission from American Society for Nutrition.
Dr Deane hypothesized that increased calorie delivery may influence outcomes in critically ill patients. He also confirmed that the ability to deliver the intervention in a blinded manner supports the development of a large multicenter double-blind RCT to determine whether critically ill patients will achieve a clinically meaningful benefit from the use of a concentrated enteral solution.
- © 2015 SAGE Publications