Summary
Serious brain- and life-threatening conditions require a high level of clinical discernment, and rapid and effective diagnostic and therapeutic action plans are needed to prevent neurologic injury, suffering, or death. Four categories that clinicians may encounter are acute cerebrovascular emergencies, pediatric neurologic emergencies, neuromuscular emergencies, and the management of seizures in the emergency department or intensive care unit setting.
- neurologic emergencies
- status epilepticus
- cerebrovascular emergencies
- intracerebral hemorrhage
- acute ischemic stroke
- neuromuscular emergencies
- pediatric neurologic emergencies
- Guillain–Barré syndrome
- myasthenia gravis
- botulism
- acute inflammatory demyelinating polyradiculoneuropathy
- ischemia
- neurology clinical trials
Diagnosis of neurologic emergencies is time-sensitive, in addition to requiring rapid diagnostic and therapeutic plans of action. Categories of emergency neurologic conditions include cerebrovascular emergencies, pediatric neurologic emergencies, status epilepticus (SE), and neuromuscular emergencies.
Cerebrovascular Emergencies
Intracerebral hemorrhage (ICH) and acute ischemic stroke represent the two most common cerebrovascular emergencies, said Jeffrey L. Saver, MD, UCLA Comprehensive Stroke Center, Los Angeles, California, USA. The burden of ICH is reflected in excessive mortality and limited recovery. Some 38% of patients have substantial hematoma expansion over the first 24 hours, which is a major mechanism of injury in ICH [Davis SM et al. Neurology. 2006].
Computed tomography (CT) is usually the initial imaging option in suspected ICH because it is highly sensitive to hemorrhage and can be performed rapidly. The goals of management of ICH are to provide general supportive care to manage the primary brain injury in the emergency department (ED) or neurologic intensive care unit (ICU) and to limit secondary brain injury. Indications for intubation are impaired airway protection or level of consciousness, signs of brainstem dysfunction, hypoxia, and risk of aspiration.
Emerging data have largely dispelled the notion of perihematoma ischemia with blood pressure lowering in ICH, said Dr Saver. The recent INTERACT2 trial demonstrated that rapid intensive blood pressure lowering had a strong trend toward rates of death or severe disability in patients with ICH [Anderson CS et al. N Engl J Med. 2013].
Seizures are more frequent in ICH than in ischemic stroke, with most occurring at ICH onset or within 24 hours. American Heart Association/American Stroke Association guidelines call for antiepileptic drug treatment of clinical ICH seizures [Morgenstern LB et al. Stroke. 2010].
ICH in any patient on warfarin with an international normalized ratio (INR) ≥ 1.5 should be considered life-threatening. Anticoagulation should be reversed in such patients, with a goal to normalize the INR (< 1.4) as quickly as possible. In patients taking direct anticoagulants, for which reversal agents are not yet available, consider 4-factor prothrombin complex concentrate, fresh frozen plasma, or both.
Proven strategies in acute ischemic stroke are recanalization, supportive care, and prevention of clot propagation, said Dr Saver. Supportive care measures include treatment of hypoxemia, maintenance of normothermia, avoidance of hyperglycemia, administration of early parenteral fluid, permissive hypertension (< 220/120 mm Hg), prophylaxis of deep vein thrombosis, and an early swallow assessment to guide oral feeding. Timely administration of intravenous (IV) recombinant tissue plasminogen (rtPA) activator increases the likelihood of improved ambulation and discharge to a more independent environment [Lees KR et al. Lancet. 2010]. Mobile stroke units offer the potential to reduce the time to thrombolysis to help achieve door-to-needle times of < 60 minutes. Direct-to-CT and ED “pit stop” protocols also facilitate early rtPA treatment to as early as 20 minutes [Ford AL et al. Stroke. 2012; Meretoja A et al. Neurology. 2012; Körhmann M et al. Int J Stroke. 2011].
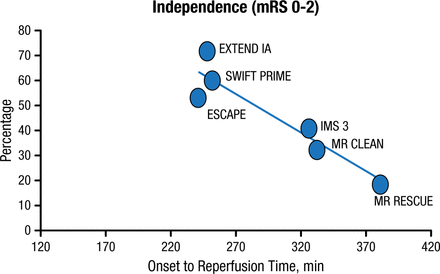
Additionally, acute mechanical recanalization is an acute stroke intervention that aims to remove the thrombus through the use of retrievers or aspirators, or to crack local atherosclerotic plaque using angioplasty and stents. After initial failures, second-generation embolectomy trials have yielded substantial reperfusion rates and positive outcomes, with the best outcomes in those trials having an onset-to-reperfusion time < 270 minutes (Figure 1).
Onset-to-Reperfusion Time and Outcome in 6 Major Thrombectomy Trials
mRS, modified Rankin scale.
Reproduced with permission from JL Saver, MD.
Pediatric Neurologic Emergencies
Ann H. Tilton, MD, Children’s Hospital, New Orleans, Louisiana, USA, followed with a discussion of pediatric neurologic emergencies, including cerebrovascular and neuromuscular disorder emergencies.
The etiology of arterial ischemic stroke in the pediatric population differs from that in adults. Sickle cell disease is the most frequent hematologic stroke-related etiology; the risk for stroke in a child with sickle cell disease is estimated to be 300 times that of an individual without the disease. Prothrombotic disorders that increase the risk of pediatric stroke are genetic thrombophilias, protein C deficiency, antiphospholipid antibodies, elevated lipoproteins, factor V Leiden mutation, antithrombin III deficiency, prothrombin gene mutation, MTHFR gene, and protein S deficiency.
With pediatric stroke of unknown etiology, aspirin should be started at 3 to 5 mg/kg/day, unless the child has sickle cell disease or ICH. Exchange transfusion and hydration with normal saline are appropriate for children with sickle cell disease. Anticoagulation with low-molecular-weight or unfractionated heparin is a rational choice if there is evidence for dissection or embolism. For children with major stroke with evidence of increased intracranial pressure and clinical deterioration, consider decompressive hemicraniotomy, advised Dr Tilton.
When a child presents with acute weakness, acute management requires the consideration of several diagnoses. One is Guillain–Barré syndrome (GBS), which represents the most common form of acute flaccid paralysis of children. The presentation in children is most often pain and difficulty walking. Criteria for ICU care include rapidly advancing weakness, flaccid paralysis, bulbar symptoms, vital capacity (VC) < 20 ml/kg, or autonomic instability. In GBS patients who have not stabilized or are unable to walk without supports, treatment of GBS is primarily IV immune globulin (IVIG) and plasma exchange.
Other disorders to consider are myasthenia gravis (MG) and status epilepticus (SE). First-line therapy for MG in pediatric patients is pyridostigmine at an initial dose of 0.5 to 1.0 mg/kg every 4 to 6 hours, up to a total daily dose of 7 mg/kg. With inadequate control, IVIG or plasmapheresis may have transient benefit, and steroids may be used in severely affected patients. Long-term control may require thymectomy.
In children with SE, placement of IV access is critical once the patient is stable. Benzodiazepines (lorazepam or diazepam) given over a minute or longer constitute initial treatment. Midazolam may be used if IV access is not available. Second-step medications include phenobarbital and valproate. In refractory SE, choices are continuous infusion of pentobarbital (first choice), midazolam, or propofol, given in an ICU setting with ventilator support.
Innovations in SE
An overview of innovations in SE over the past 5 years was provided by Nathan B. Fountain, MD, University of Virginia, Charlottesville, Virginia, USA. A retrospective study of 176 episodes of SE presenting to the ED revealed that 56% responded to first-line therapy, an additional 28% to second-line therapy, and 12% to third-line treatment, leaving 4% with refractory SE [Langer JE, Fountain NB. Epilepsy Behav. 2014].
Prehospital treatment of SE is encouraged given its safety and efficacy. The RAMPART study [Silbergleit R et al. N Engl J Med. 2012] of 448 mostly adults found that intramuscular midazolam was more effective than intravenous lorazepam for prehospital treatment of SE and was safe.
Comparative efficacy studies for second-line therapies are lacking. Valproate and phenytoin are the traditional second-line agents. Logical considerations for second-line therapy are levetiracetam and lacosamide, as both can be administered rapidly. The results from the Established Status Epilepticus Treatment Trial [ESETT; Bleck T et al. Epilepsia. 2013] should define the best second-line drug.
Drugs used in the third-line setting, when SE is generally considered refractory, are anesthetics (pentobarbital, propofol, and midazolam). Use of third-line therapy is more complicated because the character of generalized convulsive SE may change when seizure activity is prolonged, often transitioning to a form of nonconvulsive generalized SE.
In addition, questions surround the use of IV anesthetics in generalized convulsive SE. In a retrospective chart review [Sutter R et al. Neurology. 2014], IV anesthetics were associated with a 30% mortality rate compared with 10% for a group of patients receiving only parenteral antiepileptic drugs, although whether this effect is genuine is uncertain and may reflect that patients with risk factors for greater mortality were given anesthetics. IV ketamine as a third-line drug has shown interest; a retrospective multicenter study [Gaspard N et al. Epilepsia. 2013] demonstrated permanent cessation of SE in 57%, and in 12% of cases, ketamine was the last drug added, suggesting it was responsible for seizure control.
Neuromuscular Emergencies
Neuromuscular emergencies are defined as those associated with respiratory concerns or autonomic variability, stated Laurie Gutmann, MD, University of Iowa, Iowa City, Iowa, USA. Measures should include pulse oximetry, arterial blood gas, and bedside pulmonary function. Even with good respiratory function, bulbar weakness may require intubation to protect the airway, she said.
MG may present only with shortness of breath but usually has other features, such as diplopia, ptosis, dysarthria, neck flexion and extension weakness, weakness of the extremities, and dysphagia. Bedside examination includes having the patient count upward on one breath; every count of 10 is roughly 1 L of VC, and if the patient can get past 15, time is adequate for further assessment without urgently intubating. Other signs of impending respiratory compromise are use of accessory muscles for respirations and interrupted speech or shortness of breath with talking. Patients with MG require close monitoring, with intubation recommended if VC < 1 L/min or negative inspiratory pressure falls to < −20. Acute treatment consists of airway protection and IVIG or plasmapheresis.
Disorders associated with autonomic dysfunction are acquired diseases such as GBS and botulism. Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is the most common form of GBS. Patients with AIDP may have a history of parasthesias in the hands and feet with low back pain, onset of weakness in proximal legs, a preceding gastrointestinal or upper respiratory illness, and double vision. The threshold for elective intubation should be low, with VC or negative inspiratory force being low or trending downward or when the risk of aspiration is high due to poor airway protection. Early institution of IVIG infusion or plasmapheresis may help to prevent intubation or shorten the time on a ventilator.
Botulism can be differentiated from MG or AIDP by its rapid onset and a clinical history of ingestion of toxin, IV drug abuse, focal trauma with open wound, or therapeutic injection of botulinum toxin. The most common cause of death in patients with botulism is respiratory compromise, so rapid intubation and respiratory support are required. Antitoxin can be used if the illness is recognized early enough.
- © 2015 SAGE Publications