Summary
Patients with multiple sclerosis (MS) commonly suffer from fatigue that negatively affects their functioning and quality of life. Both the cause and the consequences of MS fatigue are multidimensional and require multidisciplinary treatment for successful symptom management. This article presents the results of the Efficacy of L-carnitine Versus Placebo in the Treatment of Fatigue in Multiple Sclerosis study [FACTSEP; NCT01149525]
- Neurology Clinical Trials
- Demyelinating Diseases
- Neurology
- Neurology Clinical Trials
- Demyelinating Diseases
Patients with multiple sclerosis (MS) commonly suffer from fatigue that negatively affects their functioning and quality of life. Both the cause and the consequences of MS fatigue are multidimensional and require multidisciplinary treatment for successful symptom management. The fatigue is thought to be associated with low blood carnitine levels, and some evidence suggests that carnitine might improve symptoms [Tejani AM et al. Cochrane Database Syst Rev. 2012]. The results of the Efficacy of L-carnitine Versus Placebo in the Treatment of Fatigue in Multiple Sclerosis study [FACTSEP; NCT01149525] were presented by Jean-Christophe Ouallet, MD, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France.
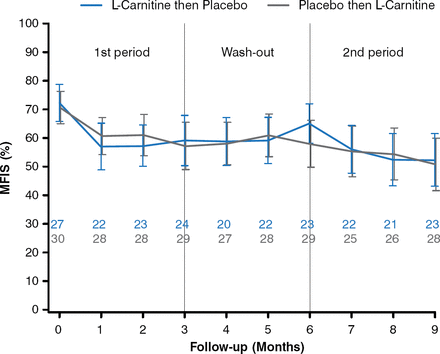
This multicenter double-blind crossover study enrolled 59 patients with relapsing-remitting MS, secondary-progressive MS, or primary-progressive MS with an Expanded Disability Status Scale (EDSS) score ≤ 6.0 and fatigue lasting > 3 months, with a global Modified Fatigue Impact Scale (MFIS) score > 45%. The patients were randomized to treatment with L-carnitine (2 g, oral solution, twice a day; n = 29) versus placebo (n = 30) for 3 months. After 3 months, all patients underwent a 3-month washout period, after which the initial L-carnitine group switched to placebo and the initial placebo group switched to L-carnitine for 3 months.
The primary outcome measure was the 21-item MFIS global score. Secondary outcome measures included the Fatigue Severity Scale (FSS), Fatigue Visual Analog Scale (VAS), physical dimension scale of MFIS, and SEP-59 Quality of Life scale. Free carnitine and acyl-carnitine serum dosages were scheduled at baseline and at the end of the study. Carnitine levels at baseline were included in the model and were tested for correlations with fatigue. Mixed linear regression models were used to assess the effect of the treatment and the treatment-period interaction. Baseline carnitine levels were included in the model and were tested for correlations with fatigue.
Of the 59 randomized patients, 57 were included in the intention-to-treat analysis. The mean patient age was 45 years; 74% of the patients were women; and the median EDSS score was 3. The baseline mean MFIS score was 71.3%, and the mean FSS score was 6.1. No significant unexpected adverse events were reported throughout the study.
For the primary outcome, there was no significant difference in the mean MFIS score between the 2 treatment groups (−0.22 points; 95% CI, −5.80 to 5.36; P = .94; Figure 1). No significant difference was observed between the groups in the FSS score (−0.10 points; 95% CI, −0.45 to 0.24; P = .55).
Effects of L-carnitine vs Placebo on Mean MFIS Score
MFIS, Modified Fatigue Impact Scale.
Reproduced with permission from JC Ouallet, MD.
For the VAS score, there was a significant difference between the groups in favor of placebo (1.43 points; 95% CI, 0.22 to 2.65; P = .02). Mild carnitine deficiency was detected in 7 patients at baseline. A more severe carnitine deficiency was found in 1 patient receiving treatment with cyclophosphamide, who received only placebo and dropped out after the washout period. L-carnitine was not effective for treating fatigue in the carnitine-deficient patients (P = .24). No evidence of efficacy was observed for the physical dimension of the MFIS, the SEP-59, the EDSS, walking ability, and other measures.
The results of this study showed that oral L-carnitine was not an effective treatment for MS fatigue when compared with placebo, even in patients with mild carnitine deficiency.
- © 2014 MD Conference Express®