Summary
The randomized phase 3 DELOS trial was designed to evaluate idebenone, a synthetic quinone compound, in Duchenne muscular dystrophy. The data showed that idebenone was safe and well tolerated, and it significantly reduced loss of respiratory function in patients aged 10 to 18 years.
- idebenone
- Duchenne muscular dystrophy
- dystrophin deficiency
- DELOS
- respiratory failure
- peak expiratory flow
- forced vital capacity
- forced expiratory volume in 1 second
- neurology clinical trials
Gunnar Buyse, MD, PhD, University of Leuven, Leuven, Belgium, presented data from a phase 3, double-blind, randomized, placebo-controlled study [DELOS; Buyse GM et al. Lancet. 2015] of the efficacy, safety, and tolerability of idebenone in patients aged 10 to 18 years with Duchenne muscular dystrophy (DMD). The results demonstrated that idebenone significantly reduced the decline of respiratory function in patients with DMD who were not receiving corticosteroids.
DMD is a devastating condition with no cure, and it is the most common type of muscular degenerative disease worldwide. Progressive respiratory failure is a major cause of morbidity in patients, and it is often the main cause of death in young adulthood.
According to Prof Buyse, in patients with DMD, dystrophin deficiency causes a calcium ion influx into muscle cells, resulting in mitochondrial dysfunction with reduced cellular energy production and increased formation of reactive oxygen species. Idebenone represents a novel treatment approach for DMD, he explained. It is a synthetic quinone compound that stimulates electron transfer and cellular energy production, and functions as an antioxidant, thereby counteracting some of the biochemical changes that occur in DMD.
Based on successful preclinical [Buyse GM et al. Eur Heart J. 2009] and phase 2 [Buyse GM et al. Pediatr Pulmonol. 2013; Buyse GM et al. Neuromusc Disord. 2011] data, Prof Buyse and colleagues therefore conducted a multicenter, international, randomized controlled phase 3 trial (DELOS) to assess the efficacy and safety of idebenone in DMD.
The study randomized and treated 64 patients aged between 10 and 18 years who were not currently receiving chronic corticosteroid treatment, of whom 92% were confined to wheelchairs.
Patients received either idebenone 900 mg/day (n = 31) or placebo (n = 33) for 52 weeks. The primary end point was peak expiratory flow (a measure of respiratory strength) as a percentage of predicted normal (PEF%p). Secondary end points included forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1).
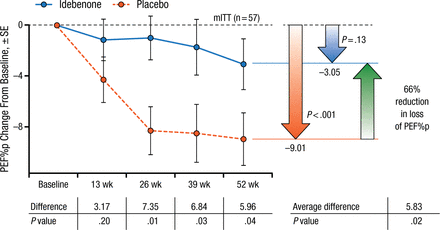
At 52 weeks, idebenone significantly reduced the decline in PEF%p compared with placebo in the modified intention-to-treat (ITT) population (–3.05 vs −9.01; P = .04; Figure 1) and the ITT population (−2.57 vs −8.84; P = .03), representing a reduction in loss of PEF%p of 66% and 71%, respectively.
Effect of Idebenone on Peak Expiratory Flow in mITT Population
mITT, modified intention-to-treat; PEF%p, peak expiratory flow as a percentage of predicted normal.
Reproduced with permission from G Buyse, MD, PhD.
Idebenone also resulted in a 37% reduction in loss of FVC as a percentage of predicted normal, although not significantly (−5.67 vs −8.95; P = .08), and a 78% reduction in loss of FEV1 as a percentage of predicted normal (−2.40 vs −10.68; P = .03) in the ITT population.
This study represents the first successful phase 3 trial in DMD, and it demonstrates significant benefit of idebenone treatment in patients with this disease. Treatment with idebenone was also safe and well tolerated, concluded Prof Buyse.
- © 2015 SAGE Publications