Summary
This article discusses the pharmacokinetic and pharmacodynamic properties of insulin, the need for fast-acting insulin in the pediatric type 1 diabetes population, and the latest approaches for accelerating the time-action profile of insulin.
- Insulin
- Diabetes Mellitus
In youth with type 1 diabetes (T1DM), increasing insulin resistance and decreased adherence to diabetes management tasks often occur during the adolescent years, leading to deterioration of glycemic control [Maffeis C et al. Pediatr Diabetes 2011]. William V. Tamborlane, MD, Yale School of Medicine, New Haven, Connecticut, USA, discussed the pharmacokinetic and pharmacodynamic properties of insulin, the need for fast-acting insulin in the pediatric T1DM population, and the latest approaches for accelerating the time-action profile of insulin.
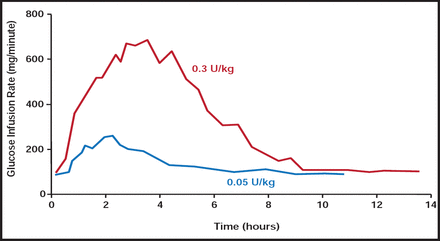
Insulin sensitivity is reduced, even in healthy lean adolescents as they progress through puberty. This insulin resistance, which appears to be related to the puberty-associated rise in growth hormone levels, is exaggerated in teenagers with T1DM, especially adolescents who are overweight or obese [American Diabetes Association. Diabetes Care 2006]. There is a need for faster-acting insulins to address the challenges of insulin resistance in the pediatric population. Adolescents with T1DM require large (ie, 0.2–0.3 U/kg) premeal bolus doses of rapid-acting insulin to overcome peripheral resistance in puberty. But, there are negative clinical consequences that are associated with this strategy. They include delayed peak, with early postmeal hyperglycemia, and prolonged duration that suppresses hepatic glucose production, causing late postmeal hypoglycemia, especially after the last meal of the day (Figure 1).
The timely delivery of insulin in doses that match the increase in blood glucose after and between meals is a therapeutic challenge [Stote R et al. J Diabetes Sci Technol 2010]. Rapid-acting insulin analogs offer the possibility of immediate preprandial or even postprandial administration in children and adolescents, who often have unpredictable sleep patterns and eating behaviors [Danne T. Diabetes Care 2007].
Dose-Dependent Pharmacodynamics of Regular Insulin.
Reproduced with permission from W. Tamborlane, MD.
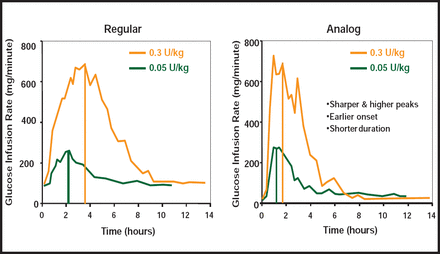
While rapid-acting insulin analogs have a more suitable pharmacokinetic and pharmacodynamic profile than soluble human regular insulin [Heller S et al. Diabetes Metab Res Rev 2011], even current insulins work too slowly and last too long for external closed-loop systems—once again resulting in exaggerated postmeal excursions, especially after breakfast, and vulnerability to late postmeal hypoglycemia, particularly after dinner.
More rapidly absorbed insulins can increase bioavailability and achieve greater within-subject consistency of bolus doses (Figure 2). Several approaches are being tested to accelerate the time-action profiles of fast-acting insulins. They include faster insulins; warming of the infusion site; coformulation with hyaluronidase; and alternate routes (microneedle infusion sets, inhaled insulin, and intraperitoneal insulin pumps).
Pharmacodynamics of Regular Versus Rapid-Acting Insulin Analogs.
Reproduced with permission from The American Diabetes Association, [Lys(B28), Pro(B29)]-human insulin. A rapidly absorbed analogue of human insulin. Howey DC et al; vol. 43, 396–402, March 1994.
The present status of these strategies varies. The first and only recombinant human hyaluronidase enzyme, rHuPH20, increases the absorption and dispersion of injected drugs. The Phase 3 Linjeta trial [NCT01067118] failed to meet criteria for noninferiority versus a comparator, and optimal temperature, timing, duration of the warming period, and the effect on infusion set age have yet to be determined for the warming device used in the study.
Each of the new approaches has shown positive results, based on pharmacokinetic and pharmacodynamic studies. However, each has its own issues regarding safety and practicality, and none has been shown to have enhanced clinical efficacy for open-loop therapy. Nevertheless, the future potential of several of these approaches in providing insulin absorption and action profiles that more closely simulate that of the normal β-cell is quite promising and complement research that is directed at the development of closed-loop insulin delivery systems.
- © 2011 MD Conference Express