Summary
A post hoc analysis of data from > 2000 patients from the CAP-START trial found that macrolide antibiotic use in hospitalized patients with community-acquired pneumonia resulted in an increased risk of cardiac events, including arrhythmia and heart failure, compared with patients who received other antibiotic therapies.
- macrolide
- antibiotic
- community-acquired pneumonia
- infectious diseases clinical trials
- bacterial infections
Macrolide antibiotic treatment of community-acquired pneumonia (CAP) was associated with an increased risk of cardiac events, such as arrhythmia and heart failure, compared with other antibiotic therapies. Douwe F. Postma, MD, University Medical Center Utrecht, Utrecht, The Netherlands, presented data from a post hoc analysis of the CAP-START trial [Postma DF et al. N Engl J Med. 2015].
International guidelines recommend macrolide antibiotics as treatment of CAP. However, an increased risk of cardiac events has been associated with macrolide antibiotic use [Schembri S et al. BMJ. 2013; Ray WA et al. N Engl J Med. 2012]. The purpose of this post hoc analysis was to determine if cardiac complications were associated with macrolide use in hospitalized patients with CAP.
In the multicenter, crossover CAP-START trial, > 2200 hospitalized patients with CAP randomly received β-lactam monotherapy, β-lactam plus macrolide therapy, or fluoroquinolone monotherapy. Each treatment was rotated through participating centers every 4 months. This post hoc analysis examined data from medical charts of new or worsening episodes of heart failure, myocardial ischemia, or arrhythmia according to prespecified criteria using a competing-risk approach. The cause-specific hazard ratio was determined using a Cox proportional hazards model, with the adjusted model accounting for 17 confounders.
Among the > 2000 evaluable patients from the CAP-START study, 15% had CAP due to Streptococcus pneumoniae infection, 33% had a history of cardiac disease, 9% had heart failure, and 15% had diabetes mellitus at baseline. In addition, upon hospitalization, the median blood urea nitrogen level was 6.4 mmol/L. The median age was 69.5 years, and 58% of patients were men. Macrolide treatment was administered to 30.8% for a minimum of 1 day.
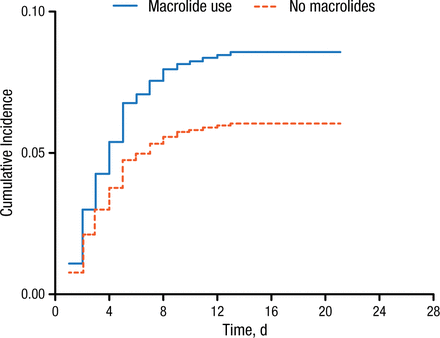
In patients who receive macrolide therapy, the cumulative incidence of cardiac events was higher than in patients who did not receive macrolides (Figure 1).
Cumulative Incidence of Cardiac Events According to Macrolide Use in Community-Acquired Pneumonia
Reproduced with permission from DF Postma, MD.
Macrolide use was associated with an increased rate (66%) and net risk (81%) of cardiac events in patients with CAP during hospitalization. Furthermore, macrolide use resulted in an increased risk for cardiac events according to cause-specific (adjusted HR, 1.66; 95% CI, 1.18 to 2.34) and subdistribution (adjusted HR, 1.81; 95% CI, 1.29 to 2.55) analyses. Specifically, macrolide use resulted in greater rates of arrhythmia (3.0% vs 2.3%) and heart failure (7.0% vs 3.7%), but not myocardial ischemia (0.3% vs 0.8%). In addition, macrolide use was associated with a greater proportion of patients experiencing in-hospital death (4.2%) compared with patients who did not receive macrolides (2.6%).
In conclusion, Dr Postma indicated that the data from this post hoc analysis of the CAP-START study suggest that macrolide use is associated with an increased risk of cardiac events, as well as in-hospital death, compared with other antibiotic therapies.
- © 2015 SAGE Publications