Summary
A novel approach to treat drug-resistant microorganisms is antimicrobial photodynamic therapy (PDT), which is under investigation in animal models. PDT involves applying light from a laser, light-emitting diode, or other light source to an infected area that has been sprayed with a pathogen-penetrating photosensitizing agent.
- Parasitic Infections
- Bacterial Infections
- Emerging Therapies
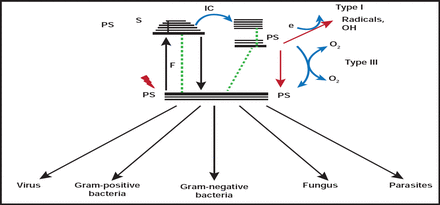
A novel approach to treat drug-resistant microorganisms is antimicrobial photodynamic therapy (PDT), which is under investigation in animal models in the lab of Michael R. Hamblin, PhD, Wellman Center for Photomedicine, Massachusetts General Hospital Boston, Massachusetts, USA. PDT involves applying light from a laser, light-emitting diode, or other light source to an infected area that has been sprayed with a pathogen-penetrating photosensitizing agent. The combination of photosensitizer and light results in the generation of cytotoxic reactive oxygen species, which kills bacteria or fungi instantly (Figure 1). “There has never been a single pathogen discovered that is resistant to photodynamic therapy,” Dr. Hamblin said.
Mechanisms of Action of Antimicrobial Photodynamic Therapy.
Reproduced with permission from M. Hamblin, MD.
PDT is safe for human tissue, because the photosensitizing agents penetrate bacteria quickly and take longer to affect eukaryotic cells. It is inexpensive and versatile and involves minimal training for staff and patients. Dr. Hamblin cites a further advantage in treating infections, such as traumatic infections and burns, since systemic antibiotics have trouble reaching damaged tissue. PDT has a broad therapeutic range, including viruses and parasites, and can reach pathogens in biofilms. In addition, Dr. Hamblin projects that PDT may be useful in the treatment of otitis media, necrotizing fasciitis, bacterial cystitis, gastric H. pylori, sinusitis, or any infection where dye and light can be infused.
Two interesting areas of PDT research are: (1) the pursuit of ideal photosensitizing agents, such as bacteriochlorins and porphycenes, and (2) assessing the effects of PDT in animal models of infection with bioluminescent organisms. Decreasing bioluminescence (correlating with decreased colony-forming units) and improved survival have been seen with burns in mouse models [Dai et al. Virulence 2001], soft tissue [Gad et al. Photochem Photobiol Sci 2004], and sepsis across a range of pathogens, including MRSA [Dai et al. Lasers in Surg and Med 2010], E. coli, Pseudomonas, Acinetobacter [Dai et al. Antimicrob Agents Chemother 2009], S. aureus [Gad et al. Photochem Photobiol Sci 2004], and Candida [Dai et al. Antimicrob Agents Chemother 2011]. PDT may also stimulate wound healing.
Scott F. Singleton, PhD, University of North Carolina, Chapel Hill, North Carolina, USA, is leading a team of researchers in the development of novel antibacterial adjunct agents that inhibit bacterial enzymes that are involved in DNA repair. Their current focus is a RecA inhibitor to combine with and potentiate the effect of DNA-damaging antibiotics. RecA protein of Escherichia coli and other anabolic enzymes are upregulated by intracellular stress that is induced by antibiotic treatment and enable bacteria to survive [Kohanski MA et al. Nature Rev Microbiol 2010]. Furthermore, bacteria that are deficient in ReA show increased susceptibility to fluouroquinolones, aminoglycosides, trimethoprim, some β-lactams, and other agents [Thi TD et al. J Antimicrob Chemother 2011; Lui et al. Antimicrob Agents Chemother 2010].
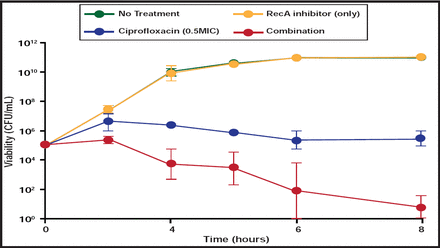
Lead candidate compound BRITE-345133, discovered by a collaborative effort between Dr. Singleton's lab and Dr. Li-An Yeh's lab at North Carolina Central University, is an allosteric inhibitor of RecA's ATPase activity. BRITE-345133 has been shown to potentiate E. coli killing by ciprofloxacin (Figure 2), which translates into a dose-dependent reduction in ciprofloxacin MIC. An added benefit of RecA inhibition and improved bacterial killing is suppression of resistance emergence. New RecA inhibitors with improved physiochemical and activity spectra are under development.
RecA Inhibitor BRITE-345133 Augments Bacterial Killing by Ciprofloxacin in vitro.
Reproduced with permission from J. Singleton, MD.
Terry Roemer, PhD, Merck Infectious Disease Research, Kenilworth, New Jersey, USA, presented his work on chemical genetic interaction networks that are aimed at uncovering targets for resensitization of methicillin-resistant S. aureus (MRSA) to β-lactam antibiotics. Plasmid-based antisense interference is a technique that impairs transcription and translation of a targeted protein and has the potential to restore the susceptibility of MRSA to β-lactam antibiotics. A wide array of genes that are involved in β-lactam tolerance processes, including early- and late-stage peptidoglycan synthesis, cell division, and cell wall biogenesis, are potential antisense targets in MRSA [Lee et al. Chem Biol. In press 2011]. Dr. Roemer shared data regarding the development of small-molecule inhibitors to resistance targets that have been identified on genetic potentiation maps, including PC190723, a novel antistaphylococcal agent that targets a component of cell division initiation, FtsZ [Haydon DS et al. Science 2008].
David Shlaes, MD, Anti-Infectives Consulting, Stonington, Connecticut, USA, offered hope in the battle against microbial resistance in the form of new β-lactamase inhibitor combinations, focusing on tazobactam, clavulanic, and a newer class of agents—avibactam (formerly called NXL-104) and MK-7655.
Pipeline agents to watch include CXA-201, a 2-to-1 combination of a new cephalosporin (CXA-101) and tazobactam. CXA-201 demonstrates good activity against strains of P. aeruginosa with diverse resistance mechanisms [Cabot G et al. ICAAC 2010] but is less active against other gram-negative pathogens, particularly those that produce extended-spectrum β-lactamases (ESBLs) [Sader HS et al. Antimicrob Agents Chemother 2011].
A 4-to-1 combination of ceftazidime and avibactam is in development for use in complicated intraabdominal abscess (IAI) and urinary tract infections (UTIs) and is expected to enter Phase 3 by early 2012. This compound shows strong activity against E. coli, Klebsiella species, and Enterobacter species, including ESBL-producing strains [Sader HS et al. ICAAC 2010]. The addition of avibactam also improves ceftazidime's activity against Pseudomonas strains with various resistance mechanisms [Eurofins Medinet Study #5006–08]. In a prospective trial for the treatment of UTI, similar efficacy was demonstrated for ceftazidime/avibactam compared with imipenem, and a greater proportion of ceftazidime-resistant E. coli responded favorably to ceftazidime/avibactam compared with imipenem (86% and 80%, respectively) [Vazquez JA et al. ECCMID 2011].
Other combinations of interest include a 1-to-1 combination of ceftaroline and avibactam that is in Phase 2 for complicated UTI and a triple combination of imipenem, cilastatin, and MK7655 that is in Phase 1. Dr. Shlaes concluded by emphasizing that he hopes drugmakers will heed the call to develop a compound, such as aztreonam (or other monobactam base agents) and avibactam (or MK7655), which should retain activity against gram-negative pathogens that bear NDM-1 or other metallo-β-lactamases.
According to Joyce Sutcliffe, PhD, Tetraphase Pharmaceuticals, Watertown, Massachusetts, USA, Streptomyces that grew on stored grain supplied ancient cultures with naturally occurring tetracycline. The 1940s and 1950s saw the development of legacy and semisynthetic tetracyclines with great oral bioavailability, and now, a technique, called total synthesis, in which the right- and left-hand segments are designed in total and then connected together, has made possible the development of new classes of tetracyclines, including 8-aminomethyl, penta/polycyclic, and 7,9 disubstituted analogs. Dr. Sutcliffe presented information on several promising agents that are in development, including orally active compounds that are effective against multidrug-resistant gram-positive and gram-negative pathogens.
PTK0796 is a new C-9-aminomethyl minocycline analog that has shown efficacy against S. aureus, E. faecalis, and E. coli in mouse models of infection [McKenney D et al. ICAAC 2003]. It is under development for intravenous and oral use in humans. PTK0796 is in Phase 2/3 development for skin and skin structure infections, with plans to study it in community-acquired pneumonia.
The 8-aminomethyl tetracycline class demonstrates activity that is comparable with tigecycline against key gram-negative pathogens, including ESBL-producing strains. From this class, TP-2758 has broad-spectrum activity, including excellent coverage of MRSA and gram-negatives, excluding Pseudomonas, and has entered Phase 1 clinical trials as an oral formulation. Other notable pipeline tetracyclines include TP-834, a pentacycline that is in development against MRSA community-acquired pneumonia, and TP-434, a 7,9 disubstituted analog with broad spectrum activity against aerobes, anaerobes, gram-positives, and gram-negatives except Pseudomonas. TP-434 is currently in Phase 2 for treating complicated IAI.
Stuart Johnson, MD, Loyola University Medical Center, Maywood, Illinois, USA, discussed a new option in the treatment of Clostridium difficile infections (CDIs). Approved in May 2011, fidaxomicin, a narrow-spectrum, nonabsorbed bactericidal RNA polymerase inhibitor that is effective against C. difficile, is the first FDA-approved agent for use in the treatment of CDI in 25 years, making it one of two approved CDI therapies, along with vancomycin. Vancomycin use is complicated by a greater-than-20% recurrence rate for CDI and has the potential to select for vancomycin-resistant strains in the gut [Kelly and LaMont. N Engl J Med 2008].
In two large, multicenter, double-blinded, randomized Phase 3 trials, fidaxomicin (200 mg BID) was shown to be noninferior to vancomycin (125 mg 4×/day) for clinical cure—88% versus 86%, respectively—in the modified intent-to-treat population. Notably, fidaxomicin treatment was associated with reduced rates of CDI recurrence compared with vancomycin (15% vs 25%; p=0.005) in the first trial [Louie TJ et al. New Engl J Med 2011]. Adverse events were similar between the two drugs, and results were similar in the second study.
The mechanism by which fidaxomicin prevents recurrence may be related to suppression of Enterobacteria overgrowth in the gut [Tannock GW et al. Microbiol 2010].
In a separate analysis of the combined Phase 3 trials of fidaxomicin- and vancomycin-treated patients, the use of concomitant antibiotics with CDI treatment was a risk factor for prolonged duration of diarrhea [Mullane KM et al. Clin Infect Dis 2011]. This is consistent with what is known about risk factors for CDI, including antibiotic use and alterations of gastrointestinal microbiota. The adverse effect of concomitant antibiotic use on cure and recurrence rates was significantly (p<0.05) more prominent among vancomycin-treated patients. Vancomycin and fidaxomicin performed similarly against infections with epidemic strain BI/NAP1/027 [Patrella L. et al. ICAAC 2011].
Looking to the future, Dr. Johnson suggested that CDI prevention strategies include immunotherapy and more effective probiotics.
With tremendous increases in the incidence of tuberculosis (TB) infection due to the HIV epidemic and the emergence of multidrug-resistant TB (MDR-TB), antituberculosis drug development has resurfaced as a major priority. Citing 2010 World Health Organization data, William Burman, MD, University of Colorado, Denver, Colorado, USA, said that a 55% increase globally in new MDR-TB cases has occurred in the past decade, with 440,000 new cases occurring each year, most of which remain undiagnosed.
Treatment of MDR-TB takes 18 to 24 months, is very expensive, and has high rates of side effects. Therefore, there is a critical need for new agents for MDR-TB.
The most advanced pipeline agent in this area is TMC207 (bedaquiline). Bedaquiline is an ATPase synthetase inhibitor with good activity against MDR-TB and has the advantage of having no activity against bacterial pathogens.
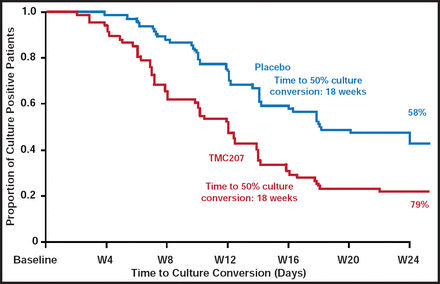
Since it interacts with rifampin, bedaquiline was evaluated as an add-on agent to optimized background therapy (excluding rifampin) in a prospective trial among patients with MDR-TB. Treatment substantially reduced (by 50%) time to sputum culture conversion (Figure 3) and was well tolerated [McNeeley DF et al. IUATLD 2010]. A FDA application is planned for 2012 for accelerated approval as an MDR treatment.
Time to Sputum Culture Conversion Among Patients with MDR-TB Treated with Optimized Background Therapy plus Placebo or TMC207.
Reproduced with permission from W. Burman, MD.
- © 2011 MD Conference Express