Summary
Using a pharmacokinetic/pharmacodynamic (PK/PD) target algorithm, the in vitro potency of CXA-201 (CXA101/tazobactam), a novel cephalosporin and b-lactamase inhibitor combination that is being developed to treat serious bacterial infections, was reported to be lower in isolates from the intensive care unit (ICU) compared with non-ICU isolates.
- Infectious Disease Clinical Trials
- Bacterial Infections
Using a pharmacokinetic/pharmacodynamic (PK/PD) target algorithm, the in vitro potency of CXA-201 (CXA101/tazobactam), a novel cephalosporin and β-lactamase inhibitor combination that is being developed to treat serious bacterial infections, was reported to be lower in isolates from the intensive care unit (ICU) compared with non-ICU isolates. This is largely driven by the differences in pathogen incidence in the two environments. Judith Steenbergen, PhD, Cubist Pharmaceuticals, Lexington, Massachusetts, USA, presented data from a study that evaluated the CXA-201 potency for pathogens that were isolated from ICU and non-ICU patients. In addition, the potency of CXA-201 against isolates from different sources of infection was evaluated.
CXA-201 is active against gram-negative pathogens, including Pseudomonas aeruginosa and Enterobacteriaceae, and select gram-positive organisms. The PK/PD parameter that was used in this study to predict efficacy was the time that was necessary to maintain concentrations of CXA-201 above the minimum inhibitory concentration (MIC) for approximately 40% to 50% of the time between dose administrations (T>MIC).
CXA-201 was tested by broth microdilution against 4134 isolates that were collected in 2008 from both ICU (n=1093) and non-ICU (n=3041) patients. A population PK model that was derived from healthy volunteers and infected patients was used to perform the Monte Carlo simulations (taking into account variability between subjects, residual variability, demographic covariates, and MIC). Target attainment rates were obtained for 1-hour infusion of 1500 mg CXA-201 every 8 hours. For pathogens with an MIC of 8 μg/mL (cutoff target), the target attainment rate was 98.2% for 40% T>MIC.
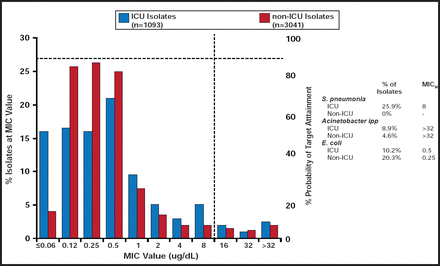
MIC90 was higher for isolates from the ICU (MIC90 =8 μg/mL) than non-ICU isolates (MIC90 =2 μg/mL). This was largely driven by differences in the percentage of Streptococcus pneumoniae, Acinetobacter spp., and Escherichia coli isolates in the ICU versus non-ICU patients (Figure 1).
Potency of CXA-201 for ICU and Non-ICU Isolates.
Reproduced with permission from J. Steenbergen, PhD.
More than 95% of all isolates had an MIC ≤8 μg/mL (8 μg/mL being the provisional breakpoint), with a range of 68% (Acinetobacter spp.) to 100% (Haemophilus influenzae). When CXA-201 potency was analyzed by site of infection, approximately 95% of all isolates had an MIC ≤8 μg/mL, with a range of 94% (blood) to 96.9% (urine).
Whether sorted by site or source, approximately 95% of isolates had an MIC value <8 μg/mL. All pathogens had an MIC90 ≤8 μg/mL except Enterobacter cloacae (88% inhibited at ≤8 μg/mL) and Acinetobacter spp. (68.5% inhibited at ≤8 μg/mL). Thus, CXA-201 is predicted to achieve excellent target attainment of 40% T>MIC against common ICU pathogens and multidrug-resistant gram-negative pathogens, including P. aeruginosa (99.3% inhibited at ≤8 μg/mL).
- © 2011 MD Conference Express