Summary
In low-risk patients with troponin-negative acute coronary syndromes undergoing ad hoc percutaneous coronary intervention, ticagrelor treatment resulted in greater reductions of platelet reactivity and a greater increase in inhibition of platelet aggregation compared with clopidogrel.
- ACS
- acute coronary syndromes
- ticagrelor
- clopidogrel
- platelet reactivity
- cardiology & cardiovascular medicine clinical trials
- interventional techniques & devices
- thrombotic disorders
Ticagrelor treatment resulted in rapid and profound reduction in platelet reactivity compared with clopidogrel in low-risk troponin-negative patients with acute coronary syndromes (ACS) who are undergoing elective or non-urgent ad hoc percutaneous coronary intervention (PCI). Roxana Mehran, MD, Mount Sinai Hospital, New York, New York, USA, presented data from an ad hoc PCI study in ACS patients [NCT01603082].
Ticagrelor is a potent P2Y12-receptor inhibitor that is currently approved by the FDA for the treatment of patients with ACS. However, patients with low-risk ACS who are troponin-negative and undergo elective or non-urgent PCI may not receive a loading dose of a P2Y12 receptor inhibitor prior to catheterization. The purpose of this study was to evaluate the effect of ticagrelor on platelet reactivity in patients with troponin-negative ACS undergoing ad hoc PCI who receive a loading dose in the catheterization laboratory immediately prior to PCI.
In this prospective, open-label, multicenter, phase 4 trial, 100 patients were randomly assigned to receive ticagrelor or clopidogrel. Patients in the ticagrelor arm (n = 51) received a 180-mg loading dose after diagnostic angiography followed by 90 mg 12 hours later. Patients in the clopidogrel arm (n = 49) received a 600-mg loading dose after diagnostic angiography. All patients received a 160- to 500-mg loading dose of aspirin followed by 75 to 100 mg of aspirin daily. Patients with ACS and ≥ 1 negative troponin test 6 to 48 hours after symptom onset were included in the study. Patients who used thienopyridine or ticagrelor within 7 days of randomization, had an indication for chronic anticoagulation, or concomitant therapy with a strong CYP3A inhibitor, substrates, or inducers were excluded.
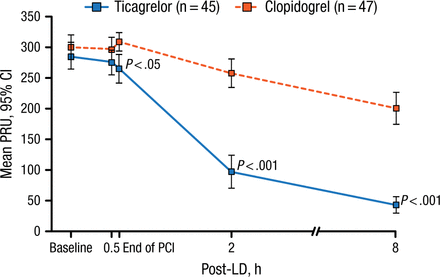
The primary end point was platelet reactivity 2 hours after dosing of the study drug, which was measured by P2Y12 reaction unit (PRU) level by the VerifyNow system. Secondary end points included PRU levels at 30 min after dosing, end of PCI, and 8 hours after dosing; percent reduction from baseline in PRU levels; and percent inhibition of platelet aggregation from baseline.
Patients who received ticagrelor demonstrated a significantly lower mean PRU level 2 hours after their loading dose compared with patients who received clopidogrel (treatment difference, −159.1; 95% CI, −194.7 to −123.5; P < .001). The significant difference in mean PRU levels occurred at the end of PCI and continued up to 8 hours following the loading dose of ticagrelor (P < .001; Figure 1). In addition, there was a significantly greater decrease in the reduction of PRU from baseline beginning at 2 hours after the loading dose compared with clopidogrel over the 8 hours (P < .001). Furthermore, inhibition of platelet aggregation was significantly greater in the ticagrelor arm compared with the clopidogrel arm across all time points.
Mean PRU Level After Treatment With Ticagrelor
LD, loading dose; PCI, percutaneous coronary intervention; PRU, platelet reaction units.
Reproduced with permission from R Mehran, MD.
The most common adverse events included chest pain, unstable angina, hypotension, dyspnea, and hematoma. Bleeding occurred in 5.9% of patients in the ticagrelor arm and 0 in the clopidogrel arm and all events were considered to be mild.
In conclusion, Dr Mehran stated that the data from this trial suggest that low-risk patients with troponin-negative ACS undergoing ad hoc PCI experienced greater platelet reactivity with ticagrelor treatment compared with clopidogrel.
- © 2015 SAGE Publications