Summary
Data from the CRASH-2 trial showed that tranexamic acid significantly reduced mortality in trauma patients, but no harm was detected. It is most effective when tranexamic acid is administered within 3 hours of the initial traumatic event. The TACTIC collaboration is currently conducting multiple preclinical and clinical studies to better manage posttraumatic hemorrhage.
- tranexamic acid
- hemorrhage
- trauma
- TACTIC
- coagulopathy
- CRASH-2
- trauma-induced coagulopathy
- hematology clinical trials
Severe trauma results in an estimated annual mortality of more than 5 million people worldwide. Uncontrolled posttraumatic bleeding is the leading cause of death among these patients, but it is potentially preventable. Appropriate management of bleeding includes measures to minimize blood loss, restore tissue perfusion, and achieve hemodynamic stability.
Ian Roberts, MB, BCh, London School of Hygiene and Tropical Medicine, London, United Kingdom, reviewed the role of tranexamic acid (TXA) in controlling surgical bleeding. Tranexamic acid is a synthetic lysine-analogue antifibrinolytic that competitively inhibits the activation of plasminogen to plasmin. At higher concentrations, it noncompetitively blocks plasmin, thus effectively inhibiting the dissolution and degradation of fibrin clots by plasmin.
In a systematic overview and cumulative meta-analysis of the literature that included 129 trials of 10 488 surgery patients [Ker K et al. BMJ. 2012], administration of TXA significantly reduced the likelihood of requiring a blood transfusion by one-third (RR, 0.62; 95% CI, 0.58 to 0.65; P < .001). However, the effect of TXA on thrombotic events and mortality was not clear. Although significantly fewer patients died in the TXA group (P = .04), the effect was not significant when the analysis was limited to the 28 trials with adequate concealment (P = .25).
Prof Roberts then reviewed results from the CRASH-2 trial [CRASH-2 Trial Collaborators. Lancet. 2010], which studied 20 211 hospitalized adult patients who were actively or at risk of bleeding after trauma. Patients were randomized to either TXA (1 g over 10 minutes followed by 1 g over 8 hours; n = 10 096) or placebo (n = 10 115). The primary outcome was death during hospitalization within 4 weeks of the initial trauma or injury. Compared with placebo, all-cause mortality was significantly reduced in the TXA arm (RR, 0.91; 95% CI, 0.85 to 0.97; P = .0035). The death due to bleeding was also significantly reduced among patients in the TXA arm (RR, 0.85; 95% CI, 0.76 to 0.96; P = .0077).
Prof Roberts focused on the hypothesis in CRASH-2 study that administering TXA early on would reduce the risk of hemorrhagic death. In fact, early treatment significantly reduced the risk of death due to bleeding (RR, 0.68; 95% CI, 0.57 to 0.82; P < .0001), whereas treatment given after 3 hours seemed to increase the risk of hemorrhagic mortality (RR, 1.44; 95% CI, 1.12 to 1.84; P = .004) [CRASH-2 Collaborators. Lancet. 2011]. He also emphasized that TXA should be in the routine trauma protocol of hospitals rather than in a massive transfusion protocol because it is a treatment for bleeding, not coagulopathy.
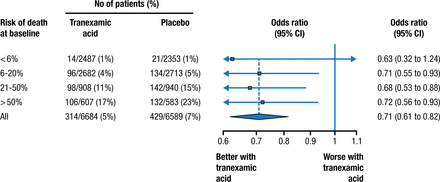
Prof Roberts then discussed that TXA is safe and efficacious for a wide range of patients [Roberts I et al. BMJ. 2012]. When the risk of death at baseline was stratified, patients who took TXA had lower mortality in every strata (Figure 1). Interestingly, there were significant reductions in arterial thrombotic events (P = .003) but not venous thrombotic events.
Death From Bleeding in Patients With Traumatic Bleeding According to Treatment With Tranexamic Acid
P = .98 for heterogeneity.
Reprinted from Roberts I et al. Effect of tranexamic acid on mortality in patients with traumatic bleeding: prespecified analysis of data from randomised controlled trial. BMJ 2012;345:e5839. With permission from the BMJ Publishing Group.
Prof Roberts summarized his talk with some recommendations for the appropriate use of TXA. In his opinion, all patients at risk of severe bleeding and who are within 3 hours of injury should receive TXA as soon as possible. He advocated that TXA treatment decisions should not depend on the results from thromboelastogram or rotational thromboelastometry, which have a low sensitivity and is a waste of time as it delays treatment.
Kenneth Mann, PhD, University of Vermont, Burlington, Vermont, USA, spoke on behalf of the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC). The goal of TACTIC is to support a single multicomponent basic collaborative research program to conduct hypothesis-driven studies of trauma-induced coagulopathy (TIC).
The incidence of acute coagulopathy of trauma shock is approximately 24% to 34% in both civilian and military casualties. It typically occurs within minutes of the original injury and is before parenteral fluid is administered for resuscitation. The incidence of coagulopathy correlates with more severe injury. Coagulopathy is associated with massive transfusion and higher morbidity. Importantly, coagulopathy increases mortality regardless of the severity score of injury. Dr Mann described that an initial abnormal prothrombin time increases the adjusted odds of dying by 35%, whereas an initial abnormal partial prothrombin time increases the odds by 326%.
According to Dr Mann, patients with acute coagulopathy treated with blood products may paradoxically become sicker. This can be caused by the transfusion of “older” packed red cells. He also reviewed some of the difficulties in studying trauma-related coagulopathy, which include the following:
The patient population is much more heterogeneous than those in other conditions.
Sample acquisition is often chaotic and unconventional.
Issue of patient consent is much more complex.
The blood condition associated with the traumatic injury itself is obscured by various replacement therapies, masking the initial coagulopathic picture.
Dr Mann then described the TACTIC team, which has been established to facilitate collaboration among scientific investigators specialized in emergency medicine and trauma surgery across the country. These collaborations are designed to share animal and in vitro models for ongoing research as well as the insight into the diagnosis and intervention of post-traumatic hemorrhage.
TACTIC has undertaken a number of projects to study (1) the effects of storage of red blood cells on thrombin generation and protein C activation; (2) the effects of trauma on the hemostatic/thrombotic response in in vivo mouse models; (3) the mechanisms contributing to altered hemostatic function in hemorrhage and trauma; (4) whether circulating histones increased thrombus stability; (5) whether small molecules generated during platelet activation influence the functions of platelets; and (6) a massive transfusion protocol that calls for the administration of rapidly defrosted plasma in the ambulance.
In his presentation, Dr Mann also highlighted several clinical trials that are conducted by the members of the TACTIC team. The PAMPer project [NCT01818427] will determine whether 2 units of AB plasma infusion before hospital admission will improve 30-day mortality in patients with hemorrhagic shock. The STAAMP trial [NCT02086500] will determine whether patients at risk of severe bleeding will have a lower 30-day mortality if 1 g of TXA was administered during air transportation. Other trials include the COMBAT [NCT01838863] and the PUPTH [NCT02303964] studies.
- © 2015 SAGE Publications