Summary
Diabetes confers approximately a 2-fold excess risk for coronary heart disease, major stroke subtypes, and deaths that are attributed to other vascular causes. In this decade, about 10% of vascular deaths in developed countries have been tributable to diabetes in adults. This article discusses the risk of diabetes in patients who are on cardioprotective medications.
- Diabetes Mellitus
- Hypertensive Disease
- Coronary Artery Disease
- Lipid Disorders
Diabetes confers approximately a 2-fold excess risk for coronary heart disease (CHD), major stroke subtypes, and deaths that are attributed to other vascular causes (Figure 1). In this decade, about 10% of vascular deaths in developed countries have been attributable to diabetes in adults. This figure corresponds to an estimated 325,000 deaths per year in high-income countries alone [The Emerging Risk Factors Collaboration. Lancet 2010]. Kausik Ray, MBChB, MD, MPhil, FRCP, FACC, FESC, St. George's University of London, London, England, discussed the risk of diabetes in patients who are on cardioprotective medications.
Lower HbA1C has Positive CV Effects.
Reprinted from The Lancet. The Emerging Risk Factors Collaboration Research Group, C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Copyright 2010. 375(9709):132–139. With permission from Elsevier.
Dysglycemia versus Diabetes and CVD Risk
Observational data suggest that the risk that is attributable to diabetes is not explained entirely by the cardiometabolic traits that cluster with diabetes, including blood glucose, suggesting that although blood glucose is used to define diabetes, glucose does not necessarily explain the excess cardiovascular (CV) risk. Furthermore, epidemiological data have shown that the CV risk that is associated with small elevations in blood glucose (6.1 to 7 mmol/L) increases the CV risk by about 17% compared with a reference group (3.5 to 5.6 mmol/L). In contrast, among individuals who are not known to have a clinical diagnosis of diabetes but with fasting glucose levels >7 mmol/L (the cutoff point for diabetes), the risk is increased by 78%.
Oral Hypoglycemics
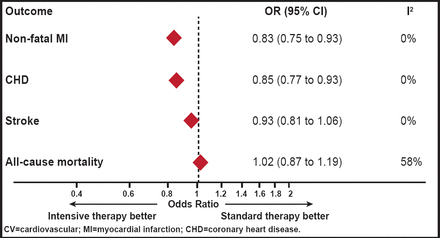
A meta-analysis showed that intensive glycemic control significantly reduces coronary events without an increased risk of death compared with standard glycemic control in 33,040 patients across five randomized, controlled trials [Ray KK et al. Lancet 2009]. Intensive glycemic control reduced HbA1C by 0.9% over that achieved with standard treatment, resulting in a 17% reduction in events of nonfatal MI (OR, 0.83; 95% CI, 0.75 to 0.93) and a 15% reduction in CHD events (OR, 0.85; 95% CI, 0.77 to 0.93). Intensive glycemic control had no significant effect on stroke (OR, 0.93; 95% CI, 0.81 to 1.06) or all-cause mortality (OR, 1.02; 95% CI, 0.87 to 1.19; Figure 1).
The DREAM (Diabetes REeduction Assessment with Ramipril and Rosiglitazone Medication) Trial investigated the ability of rosiglitazone to prevent type 2 diabetes in individuals who are at high risk of developing the condition [Gerstein HC et al. Lancet 2006]. The primary outcome was a composite of incident diabetes or death.
At the end of the study, 306 (11.6%) individuals who received rosiglitazone and 686 (26.0%) who received placebo developed the composite primary outcome (HR, 0.40; 95% CI, 0.35 to 0.46; p<0.0001); 1330 (50.5%) of those in the rosiglitazone group and 798 (30.3%) in the placebo group became normoglycemic (HR, 1.71; 95% CI, 1.57 to 1.87; p<0.0001). CV event rates were similar in both groups, although 14 (0.5%) participants in the rosiglitazone group and 2 (0.1%) in the placebo group developed heart failure (p=0.01). Taken together, data from observational and intervention studies suggest that oral hyperglycemics reduce the incidence of type 2 diabetes but not cardiovascular disease (CVD).
Type 2 diabetes and dysglycemia are not one and the same, and the CV risk with dysglycemia is modest.
Antihypertensive Drugs
A 2000 article in the New England Journal of Medicine [Gress TW et al.] showed that subjects who were taking thiazide diuretics were not at greater risk for the subsequent development of diabetes than those with hypertension who were not receiving any hypertensive therapy (relative hazard, 0.91; 95% CI, 0.73 to 1.13). Likewise, subjects who were taking ACE inhibitors and calcium channel antagonists were not at greater risk than those who were not taking any medication.
In contrast, those with hypertension who were taking β-blockers had a 28% higher risk of subsequent diabetes (relative hazard, 1.28; 95% CI, 1.04 to 1.57). The authors concluded that the increased risk of diabetes with the use of β-blockers must be weighed against the proven benefits of the drugs in reducing the risk of CV events [Gress TW et al. N Engl J Med 2000].
Since then, a systematic review in Lancet [Elliott WJ, Meyer PM] has shown the risk of incident diabetes in other classes of antihypertensives. The association of antihypertensive drugs with incident diabetes was lowest for ARBs and ACE inhibitors, followed by CCBs and placebo, β-blockers, and diuretics in descending order (Table 1).
Association of Antihypertensive Drugs with Incident Diabetes.
Statins
Findings from individual studies conflict on the risk of incident diabetes mellitus in patients who are being treated with statins. In a meta-analysis, Sattar et al. [Lancet 2010] found that statin therapy was associated with a 9% increased risk for incident diabetes in 90,000 patients (OR, 1.09; 95% CI, 1.02 to 1.17). Meta-regression showed that risk for diabetes with statins was highest in trials with older participants, but neither baseline body mass index nor change in low-density lipoprotein cholesterol (LDL-C) concentrations accounted for residual variation in risk.
Treatment of 255 (95% CI, 150 to 852) patients with statins for 4 years resulted in 1 extra case of diabetes, indicating that statin therapy is associated with a slightly increased risk of diabetes development, but the risk is low both in absolute terms and when compared with the reduction in coronary events. The authors recommended that clinical practice in patients with moderate or high CV risk or existing CVD should not be changed [Sattar N et al. Lancet 2010].
A meta-analysis by Preiss et al. [JAMA 2011] found that intensive-dose statin therapy was associated with an increased risk of new-onset diabetes compared with moderate-dose statin therapy. For every 3 CVD events that were prevented, there was 1 extra case of diabetes, suggesting that the benefits outweigh the risks. This is further supported by the Cholesterol Treatment Trialists' (CTT) Collaborators [Kearney PM et al. Lancet 2008] meta-analysis among diabetics, which showed a 9% proportional reduction in all-cause mortality per mmol/L reduction in LDL-C of participants with diabetes (rate ratio [RR], 0.91; 99% CI, 0.82 to 1.01; p=0.02), which was similar to the 13% reduction in those without diabetes (RR, 0.87; 95% CI, 0.82 to 0.92; p<0.0001).
This finding reflected a significant reduction in vascular mortality (RR, 0.87; 95% CI, 0.76 to 1.00; p=0.008), with no effect on nonvascular mortality (RR, 0.97; 95% CI, 0.82 to 1.16; p=0.7) in subjects with diabetes. There was a significant 21% proportional reduction in major vascular events per mmol/L reduction in LDL cholesterol in people with diabetes (RR, 0.79; 95% CI, 0.72 to 0.86; p<0.0001). This was similar to the effect that was observed in those without diabetes (RR, 0.79; 95% CI, 0.76 to 0.82; p<0.0001). The authors concluded that statin therapy should be considered for all diabetic individuals who are at sufficiently high risk of vascular events.
Statins increase the risk of dysglycemia but reduce the risk of CVD. As the relative benefit on CVD reduction increases over time and as absolute risk of CVD increases over time, if for no other reason than age, the absolute benefit of statins also increases over time by prevention of second and third events and as absolute risk increases [LaRosa JC et al. Am J Cardiol 2010]. Statins, niacin, thiazides, and β-blockers are associated with dysglycemia in a dose-dependent manner. However, short-term consequences are offset by CVD benefits.
- © 2011 MD Conference Express®