Summary
Developing new therapies for drug addiction depends on understanding the underlying mechanisms for the process of addiction. Changes in dopamine receptors and brain function are critical in addiction. This article discusses the latest advances in addiction research.
- Substance-Related Disorders
- Psychiatry
- Substance-Related Disorders
- Psychiatry
Developing new therapies for drug addiction depends on understanding the underlying mechanisms for the process of addiction. Changes in dopamine receptors and brain function are critical in addiction. Nora D. Volkow, MD, National Institute on Drug Abuse, Bethesda, Maryland, USA, discussed the latest advances in addiction research.
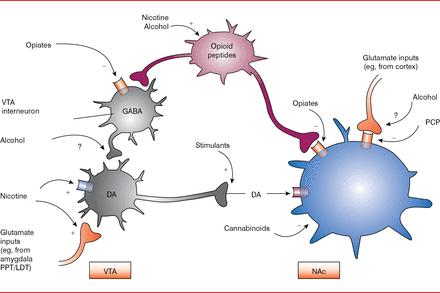
All drugs that are abused, including nicotine and alcohol, cause an increase in dopamine levels in the nucleus accumbens, albeit by differing mechanisms (Figure 1) [Nestler EJ. Nat Neurosci 2005]. For example, within 1 hour after nicotine intake, a substantial increase in the percentage of basal dopamine occurs, which gradually decreases over several hours [Di Chiara G et al. Neuropharmacology 2004]. A similar trend is seen after methamphetamine intake. In addition, drugs activate the glutamate receptors more potently, with longer-lasting effects, than do normal stimulants, such as food and social interactions. This is termed supraphysiological stimulation of the receptors and results in downstream neuronal adaptations, such as dopaminergic transmission and enhancement of AMP and N-methyl-D-aspartate.
Dopamine Modulation by Abused Drugs
DA=dopamine; GABA=gamma-aminobutyric acid; LDT=lateral dorsal tegmentum; NAc=nucleus accumbens; PCP=phencyclidine; PPT=peduncular pontine tegmentum; VTA=ventral tegmental area of Tsai.
Reproduced from Nester El. Is there a common molecular pathway for addiction? Nat Neurosci 2005;8(11):1445–1449. With permission from the Nature Publishing Group.
A study of how dopamine levels increase by intravenous methylphenidate (MPH) in humans showed that MPH binds to the dopamine transporter, inhibiting its activity, which allows dopamine to build up within the synaptic cleft, thereby creating an amplification effect of the original stimulator [Volkow ND et al. J Pharmacol Exp Ther 1999]. In addition, the perception of feeling a high was associated with higher levels of dopamine. However, this increase in dopamine is not observed with oral MPH, which is likely a result of the rate of the dopamine increase. Intravenous MPH causes a rapid rise in dopamine levels, which mimics dopamine cell signaling, whereas oral MPH causes a slower rise in dopamine [Volkow ND et al. Proc Natl Acad Sci USA 2011]. A slow rise in dopamine mimics a chronic dopamine signaling effect, which plays a role in cognitive, motivational, and motoric systems and does not produce a high feeling. Interestingly, phasic dopamine primarily stimulates dopamine 1 receptors (D1Rs), whereas tonic dopamine stimulates dopamine 2 receptors (D2Rs). Therefore, the process of drug reward requires that D1Rs are stimulated, and it is optimal if both D1Rs and D2Rs are stimulated. Dr. Volkow suggested that this is why intravenous and inhalation modes of drug administration are more addictive—they cause a rapid rise in dopamine, which mimics the endogenous process of reward.
In cocaine abusers, intravenous administration of MPH does not cause an increase in dopamine to the same extent that it does in normal controls (p<0.001) [Volkow ND et al. Proc Natl Acad Sci USA 2011]. Even when cocaine abusers are provided with cocaine using-associated cues, the effect of an MPH injection on dopamine is minimal compared with that of normal controls (p<0.001) [Volkow ND et al. Mol Psychiatry. In press]. Interestingly, in rats that receive chronic treatment with cocaine, there is substantial attenuation of dopamine signaling through D2Rs, thereby showing a conditioned response [Volkow ND et al. Neurobiol Learn Mem 2002].
The D1Rs and D2Rs are downregulated in the presence of excess dopamine and upregulated in the presence of inadequate dopamine. In one study, mice that received adenovirus carrying the D2Rs had an increase in D2Rs levels that corresponded with a decrease in self-administered alcohol intake [Thanos PK et al. J Neurochem 2001]. A similar effect is observed in rats, where upregulation of D2Rs corresponds with a decrease in cocaine self-administration.
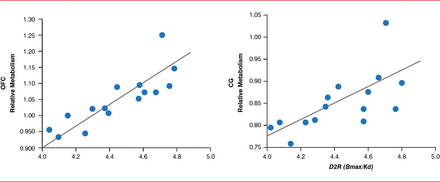
Drug addiction also appears to affect brain function, as measured by glucose metabolism evaluated by imaging studies. In an imaging study, lower D2R levels were associated with less activity in the prefrontal cortex [Volkow ND et al. Proc Natl Acad Sci USA 2011]. Dr. Volkow highlighted that even at baseline, without stimulation with a drug, drug abusers demonstrated decreased activity in the prefrontal cortex. In another study, patients who did not abuse alcohol but had a family history of alcoholism demonstrated greater levels of D2Rs in the prefrontal cortex, which was associated with greater activity as measured by glucose metabolism in that area (Figure 2) [Volkow ND et al. Arch Gen Psychiatry 2006]. Dr. Volkow suggested that this may be a mechanism for why some people are vulnerable to addiction, in terms of increased compulsivity and impulsivity.
Family History of Alcoholism Affects Brain Function
CG=anterior cingulate gyrus; D2R=dopamine 2 receptor; OFC=orbitofrontal cortex.
Reproduced from Volkow ND et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry 2006;63(9):999–1008. With permission from the American Medical Association.
It has been observed that rats that are chronically exposed to alcohol, cocaine, or opiates are hypersensitive to other stressors, which in turn lead them to further compensate for that stress with the drug. In addition, several genomewide association studies found that nicotine receptors a4, α3, and b4 were associated with nicotine dependence [Berrettini W et al. Mol Psychiatry 2008; Schlaepfer IR et al. Biol Psychiatry 2008; Bierut LJ et al. Human Mol Genet 2007]. These subunits of the nicotine receptor are expressed in the medial habenula. Interestingly, a study in monkeys found that the habenula is involved in a negative reward process, in which increased activity in the habenula results in no reward by inhibiting dopamine neurons [Kimura M et al. Nat Neurosci 2007]. Dr. Volkow noted that these data suggest that smokers may be ingesting nicotine to inhibit the activity of the habenula, thereby preventing the habenula from affecting dopamine activity.
Dr. Volkow pointed out that drug addiction is a dynamic disease that occurs in cycles. Exposure to the drug in combination with stressors results in the anticipation and craving for the drug [Koob GF, Volkow ND. Neuropsychopharmacology 2010]. The craving leads to the binge or intoxication phase. Therefore, there are periods of abstinence, craving, and intoxication. The abstinence or negative affect stage, before craving, is the point at which patients can be engaged. In one study, cocaine users were told that they would be receiving the drug but that they should refuse it [Volkow ND et al. Annu Rev Pharmacol Toxicol 2012]. They were able to not only inhibit the nucleus accumbens but also activate the right inferior prefrontal cortex, which Dr. Volkow called the “brake system” of the brain. However, Dr. Volkow pointed out that this cannot be done when patients are in a state of intoxication. Therefore, patients have to be taught to predict when exposure will occur so that they can prevent further use of the drug.
Another approach to treat addicts is the use of a medication, such as MPH, to improve the function of the prefrontal cortex to allow them to better follow an intervention. In a study of cocaine abusers, administration of MPH resulted in increased activity in the prefrontal cortex, which was associated with improvement in Stroop test scores [Goldstein RZ, Volkow ND. Neuropsychopharmacology 2011]. Other potential treatments for addiction include repetitive transcranial magnetic stimulation or direct current stimulation for tobacco addiction [Wing VC et al. Brain Stimulation 2013] and a vaccine against cocaine and nicotine addiction [Shen XY et al. Clin Pharmacol Ther 2012]. A mechanism behind addiction vaccines is that vaccine-stimulated antibodies could delay the absorption of the drug across the blood-brain barrier. A Phase 3 trial of a vaccine against nicotine addiction was performed. Dr. Volkow commented that results of the trial were disappointing but likely because only a small number of patients were able to achieve an effective titer. Therefore, the challenge of developing an effective vaccine is ensuring that patients are antigenic.
A major challenge for the National Institute of Drug Addiction is the changing perception of marijuana as a nonharmful drug. For the last 3 years, more children in Grade 12 use marijuana than smoke cigarettes [Johnston LD et al. Monitoring the Future National Results on Drug Use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan. 2014]. This is important because persistent cannabis use is associated with mental illness and lower IQ. Discontinuation of cannabis use did not resolve the loss in IQ. Indeed, chronic use of cannabis is associated with neuropsychological decline, beginning in childhood and continuing to midlife [Meier MH et al. Proc Natl Acad Sci USA 2012]. In another study, frequent use of cannabis in adolescence was associated with loss of axonal fiber connectivity by up to ∼90%, particularly in the hippocampus, hippocampal commissure, and splenium [Zalesky A et al. Brain 2012]. Dr. Volkow pointed out that the hippocampus is involved in intelligence, so this finding may explain why a decrease in IQ is associated with cannabis use.
Advances in addiction research help uncover the underlying mechanisms related to addictive behaviors and the process of addiction as a result of drug use. New strategies for the treatment of addiction are on the horizon but require further study.
- © 2014 MD Conference Express®