Summary
A multinational, randomized, double-blind, placebo-controlled Efficacy Study of Vortioxetine on Cognitive Dysfunction in Adult Patients With Major Depressive Disorder (MDD) [FOCUS; NCT01422213] has demonstrated the drug's efficacy in improving cognitive function and lessening depression symptoms [APA 2014 (poster NR6-114)].
- Psychopharmacology
- Psychiatry Clinical Trials
- Mood Disorders
- Psychopharmacology
- Psychiatry
- Psychiatry Clinical Trials
- Mood Disorders
A multinational, randomized, double-blind, placebo-controlled Efficacy Study of Vortioxetine on Cognitive Dysfunction in Adult Patients With Major Depressive Disorder (MDD) [FOCUS; NCT01422213] has demonstrated the drug's efficacy in improving cognitive function and lessening depression symptoms. The poster chronicling the study was presented by Roger S. McIntyre, MD, University Health Network, University of Toronto, Toronto, Ontario, Canada [APA 2014 (poster NR6-114)].
Vortioxetine is a novel multimodal antidepressant that functions as a human 5-HT3A and 5-HT7 receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist, and an inhibitor of the serotonin transporter. It is thought to act directly on the serotonin receptor and on the inhibition of serotonin reuptake. Vortioxetine was approved in 2013 for the treatment of MDD by the US Food and Drug Administration.
The FOCUS study comprised secondary analyses of the effect on specified end points of acute treatment with vortioxetine doses of 10 mg/day (n=195) and 20 mg/day (n=207) versus placebo (n=196) for 598 adults with recurrent moderate-to-severe MDD. The patients were aged ≥18 years and ≤65 years, diagnosed with recurrent MDD according to Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision; DSM-IV-TR) with a current depressive episode lasting 3 months or longer, and a Montgomery-Asberg Depression Rating Scale (MADRS) total score ≥26 at screening and baseline.
The primary outcome was the effect of cognitive assessments. Secondary outcomes were changes in depression symptom severity from baseline at Weeks 1, 4, and 8 in MADRS total score, MADRS response and remission, Clinical Global Impression-Severity of Illness (CGI-S), and the Clinical Global Impression-Improvement (CGI-I) scores.
The baseline characteristics of patients in the three study arms were similar. The mean baseline MADRS scores were indicative of moderate to severe depression in the patients (Table 1).
Baseline Patient Characteristics
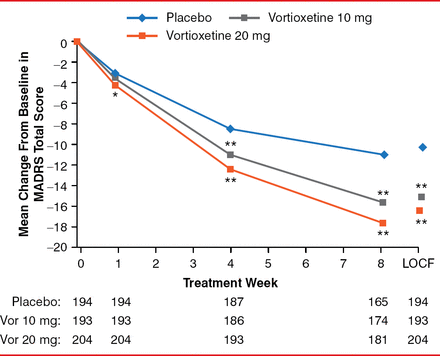
After 8 weeks, the mean MADRS decreased (improved) by 10.9, 15.6, and 17.6 points for the placebo, vortioxetine 10-mg, and vortioxetine 20-mg arms, respectively. The differences between the vortioxetine doses and placebo were significant (both p<0.001). The difference in the mean change from baseline to Week 8 in the MADRS total score was −4.7 and −6.7 for 10-mg/day and 20-mg/day vortioxetine, respectively (both p<0.001; Figure 1).
Estimated MADRS Total Scores From Baseline to Week 8 and LOCF
*p<0.01, **p<0.001 vs placebo.
LOCF=last observation carried forward; MADRS=Montgomery-Asberg Depression Rating Scale.
Reproduced with permission from RS McIntyre, MD.
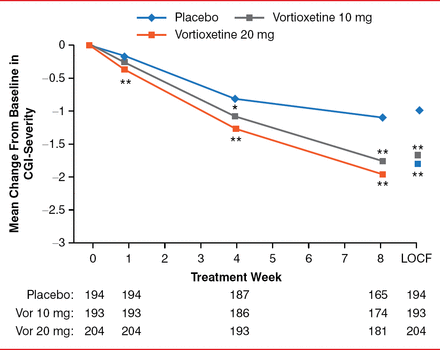
Vortioxetine 10 mg was distinguished from placebo on six of the 10 MADRS items at Week 4 and all 10 items at Week 8. Vortioxetine 20 mg was distinguished from placebo on 3 MADRS items at Week 1, 9 items at Week 4, and all 10 items at Week 8. The improvement in the rating of depression was manifest clinically, as indicated by improvement in CGI-S of −0.08 and −0.18 at Week 1 (p=0.077 and p<0.001, respectively); −0.27 and −0.43 at Week 4 (p=0.004 and p<0.001, respectively); and −0.65 and −0.85 at Week 8 (both p<0.001) for vortioxetine 10 and 20 mg versus placebo, respectively (Figure 2).
CGI-S Scores and LOCF
*p<0.01, **p<0.001 vs placebo.
CGI-S=Clinical Global Impression-Severity of Illness; LOCF=last observation carried forward.
Reproduced with permission from RS McIntyre, MD.
Vortioxetine was well tolerated. Most frequent adverse effects for the placebo, vortioxetine 10-mg, and vortioxetine 20-mg arms were nausea (4.1%, 16.4%, and 20.8%, respectively) and headache (7.1%, 8.2%, and 12.6%, respectively).
The secondary analyses establish the efficacy of the two vortioxetine doses on lessening depression in patients with MDD.
- © 2014 MD Conference Express®