Summary
This article presents results from the first half of the randomized controlled International Study to Predict Optimized Treatment in Depression [iSPOT-D; NCT00693849]. The study demonstrated that, for patients with major depressive disorder, escitalopram, sertraline, and venlafaxine extended release produced similar treatment response rates, with mild and similar side effects.
- Mood Disorders
- Psychopharmacology
- Psychiatry Clinical Trials
- Psychiatry
- Mood Disorders
- Psychopharmacology
- Psychiatry Clinical Trials
Radu V. Saveanu, MD, Leonard M. Miller School of Medicine, University of Miami, Miami, Florida, USA, presented results from the first half of the randomized controlled International Study to Predict Optimized Treatment in Depression [iSPOT-D; NCT00693849]. The study demonstrated that, for patients with major depressive disorder (MDD), escitalopram, sertraline, and venlafaxine extended release (XR) produced similar treatment response rates, with mild and similar side effects.
Although antidepressant medications (ADMs) are effective, their benefit could be enhanced by identifying pretreatment clinical or neurobiological features that predict response versus nonresponse to treatment, as well as features or moderators that help identify which specific treatment is the best match for a particular patient.
With this in mind, Dr. Saveanu and colleagues conducted the multiphase, multisite, randomized controlled iSPOT-D trial. This ongoing real-world effectiveness trial, with no placebo arm, was designed to identify genetic, physical, and psychological markers that predict specific response to a range of ADMs in a large group of outpatients diagnosed with MDD. Focusing on outcomes that may affect how personalized medicine is implemented in depression, the study was designed to identify predictors and moderators of outcomes in order to change how ADMs are selected.
To be included in the study, participants were required to be 18 to 65 years old, meet the DSM criteria for MDD, and score ≥16 on the Hamilton Rating Scale for Depression, 17-Item (HAM-D-17).
Exclusion criteria included suicidal ideation or planning, contraindication to study ADMs, recurrent or current substance dependence, and other mental disorders.
Outcome measures were obtained 8 weeks after ADM treatment. The study's primary outcome was rate of treatment response (defined by ≥50% improvement) and remission (defined by score ≤7) using the clinician-rated HAM-D-17 score. Secondary outcomes included rate of treatment response (defined by ≥50% improvement) and remission (defined by score ≤5) using the self-reported Quick Inventory of Depressive Symptomatology, 16-Item (QIDS-SR16) score; functional capacity; and side effect burden to the three ADMs.
In Phase 1 of the trial, participants (n=1008; mean age 37.8 years; 57% female) were randomly assigned to receive escitalopram (10 mg/day; maximum 20 mg/day), sertraline (50 mg/day; maximum 200 mg/day), or venlafaxine XR (75 mg/day; maximum 225 mg/day), the three first-line ADMs prescribed worldwide.
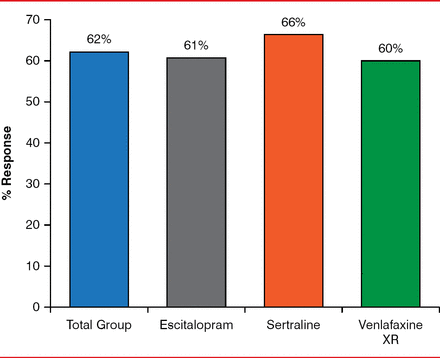
HAM-D-17 response rates were similar and consistent across the escitalopram, sertraline, and venlafaxine arms (61% vs 66% vs 60%, respectively; Figure 1), as were HAM-D-17 remission rates (48% vs 46% vs 42%, respectively). QIDS-SR16 response rates (55.7% vs 55.6% vs 48.4%) and remission rates (41.0% vs 38% vs 33.8%, respectively) were also similar across the three arms. Side effects were also mostly mild, and they occurred in a similar number of participants among the three groups (85% vs 80% vs 79%, respectively).
HAM-D-17 Response Rates Across the Three ADM Arms (defined by ≥ 50% improvement)
HAM-D-17=Hamilton Rating Scale for Depression, 17-Item; XR=extended release.
Reproduced with permission from RV Saveanu, MD.
The results of the first half of this study indicate that escitalopram, venlafaxine, and sertraline produce similar and consistent response rates in patients with MDD, with similar and mild side effects. These findings even the clinical research playing field for identifying predictors and moderators of response that can be translated into patient care, Dr. Saveanu concluded.
- © 2014 MD Conference Express®