Summary
Although no drugs are currently approved for treatment of borderline personality disorder (BPD), studies have shown the benefit of antipsychotic agents for this indication, particularly when symptoms of mood instability, anger and irritability, and self-harm are prominent. Open-label studies have demonstrated that the atypical antipsychotic drug quetiapine is efficacious and well tolerated in treating these patients. This article presents results of a placebo-controlled trial demonstrating that low doses of long-acting quetiapine significantly improve symptoms in patients with BPD.
- Psychopharmacology
- Psychiatry Clinical Trials
- Personality Disorders
- Psychopharmacology
- Psychiatry Clinical Trials
- Psychiatry
- Personality Disorders
Donald W. Black, MD, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA, presented results of a placebo-controlled trial demonstrating that low doses of long-acting quetiapine significantly improve symptoms in patients with borderline personality disorder (BPD).
Although no drugs are currently approved for treatment of BPD, studies have shown the benefit of antipsychotic agents for this indication, particularly when symptoms of mood instability, anger and irritability, and self-harm are prominent. Open-label studies have demonstrated that the atypical antipsychotic drug quetiapine is efficacious and well tolerated in treating these patients.
Dr. Black and colleagues conducted a randomized controlled trial (RCT) to investigate the efficacy and tolerability of extended-release quetiapine in the treatment of BPD [Black DW et al. Am J Psychiatry 2014. In press; NCT00880919]. To be included in the study, patients were required to be from 18 to 45 years old, meet DSM-IV criteria for BPD, and show a score of ≥9 on the Zanarini Rating Scale for BPD (ZAN-BPD) at screening.
Exclusion criteria included patients with various psychiatric and nonpsychiatric conditions, such as comorbid current major depressive disorder or substance abuse, and pregnant women. Those with a history of nonresponse to an atypical antipsychotic agent were also excluded.
A total of 95 patients were randomly assigned to groups receiving quetiapine 150 mg/day (n=33), quetiapine 300 mg/day (n=33), or placebo (n=29). All patients received a 50-mg starting dose on Day 1, which increased to 150 mg/day after 1 week; patients in the 300-mg/day arm were changed to that level after 4 weeks. The study comprised three treatment phases: screening (Visits 1 and 2), treatment (Visits 2 to 10), and discontinuation (Visits 10 and 11).
Primary outcome was ZAN-BPD total score. Secondary outcomes included the Borderline Evaluation of Severity over Time (BEST) index of BPD symptoms and ZAN-BPD subscales.
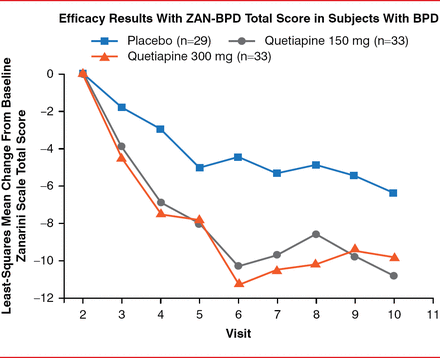
Patients in all arms of the study experienced symptom improvement, as demonstrated by reduced ZAN-BPD total score (Figure 1). In both quetiapine arms, improvement was greatest from baseline (Visit 2) to Visit 6. The improvement was only significant (p=0.031) in the quetiapine 150-mg/day arm compared with placebo, however.
Changes in ZAN-BPD Total Score
BPD=borderline personality disorder; ZAN-BPD=Zanarini Rating Scale for Borderline Personality Disorder.
Reproduced with permission from DW Black, MD.
All secondary outcomes improved significantly in both quetiapine groups compared with the placebo group, except the improvement in the Young Mania Rating Scale score in the 300-mg/day group, which missed significance (p=0.06).
Eighty-eight percent of patients reported at least one adverse event (AE), with a higher risk of occurrence in the quetiapine 300-mg/day arm. The most common AEs in this group were sedation (HR, 2.16), appetite change (HR, 3.89), and dry mouth (HR, 16.8). The discontinuation rate in the study was high (33%). By 8 weeks, 42% of patients in the 300-mg/day group had discontinued, compared with 33% of the 150-mg/day group and with 20% of those receiving placebo. Sedation was a predictor of discontinuation (HR, 1.77).
Dr. Black concluded that, despite the high discontinuation rate, which is not uncommon in trials in BPD, the results of this study demonstrate efficacy of low-dose quetiapine in treating the symptoms of BPD. He recommended that future RCTs involving larger patient numbers and active comparators will further understanding of the effectiveness and safety of atypical antipsychotic drugs in patients with BPD.
- © 2014 MD Conference Express®