Summary
Several new therapies have shown promise in the treatment of adult and pediatric bipolar depression and obsessive compulsive disorder (OCD). This article discusses next generation in treatments for OCD, new data for pharmacotherapy of bipolar depression, as well as specific trials..
- Anxiety Disorders
- Mood Disorders
- Psychiatry
- Anxiety Disorders
- Mood Disorders
Several new therapies have shown promise in the treatment of adult and pediatric bipolar depression and obsessive compulsive disorder (OCD). Zafar Sharif, MD, Harlem Hospital Center, New York, New York, USA, presented data from the Efficacy of Cognitive Remediation in Patients With Schizophrenia or Schizoaffective Disorder Stabilized on Lurasidone study [NCT01173874].
In this multicenter Phase 3 trial, 133 patients diagnosed with schizophrenia or schizoaffective disorder for > 1 year received 40 to 160 mg of lurasidone daily for up to 8 weeks and then were randomly assigned to receive cognitive remediation plus lurasidone or computer games plus lurasidone for up to 26 weeks. Patients aged 18 to 55 years were included if a change in medication was clinically warranted, their Wechsler Test of Adult Reading raw score was > 12, and they were able to complete a MATRICS Consensus Cognitive Battery (MCCB) at Week 0. Exclusion criteria included documented learning disability, hearing or visual impairment, current or history of treatment with clozapine, alcohol or substance abuse or dependence, or recent exposure to cognitive remediation. The primary outcomes were University of California, San Diego, Performance-Based Skills Assessment (UPSA)-B total score and Positive and Negative Syndrome Scale (PANSS).
The trial was completed by 76 patients (33 in cognitive remediation, 43 in computer game). In the open-label, stabilization phase during the first 8 weeks of lurasidone treatment, PANSS total, positive, and negative scores decreased, and MCCB composite and domain T-scores increased. In addition, patients demonstrated a significant increase in UPSA-B total score (p<0.001).
In patients who underwent the cognitive remediation, the MCCB cognitive composite score significantly increased from Week 20 to Week 32 (p<0.05); however, there was no significant difference between the cognitive remediation and computer game arms at both Week 20 and Week 32. Similarly, there was no significant difference in change in UPSA-B score for both the cognitive remediation and computer game arms from Week 20 to Week 32.
Dr. Sharif concluded that the results of this preliminary analysis of the trial indicate that cognitive remediation does not appear to be superior to nonspecific computer activity. However, the small sample size is a limitation of the study.
Carolyn Rodriguez, MD, PhD, Columbia University, New York, New York, USA, presented the next generation in treatments for OCD. Patients with OCD have greater glutamate levels in their cerebrospinal fluid [Chakrabarty K et al. Neuropsychopharmacology 2005] and an association between genes involved in the glutamate system and OCD has been suggested [Stewart SE et al. Am J Med Genet B Neuropsychiatr Genet 2007; Arnold PD et al. Arch Gen Psychiatry 2006; Dickel DE et al. Arch Gen Psychiatry 2006]. Several agents target the glutamate system: minocycline, ketamine, riluzole, and memantine.
Minocycline enhances the transport of glutamate form the presynaptic cleft to neighboring astrocytes or glia. In an open-label trial, 9 patients aged 18 to 65 with primary OCD stable on a serotonin reuptake inhibitor for at least 12 weeks received 50 mg of minocycline BID for 3 days, then 100 mg BID for 12 weeks [Rodriguez CI et al. J Clin Psychiatry 2010]. Overall, there was no significant difference in the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) over the 12-week period. However, patients who did respond to minocycline demonstrated a 45% decrease in Y-BOCS over 12 weeks. Interestingly, patients who responded to minocycline had childhood onset OCD, whereas nonresponders had adult onset OCD.
A case report demonstrated that intravenous infusion of ketamine in a patient with treatment-resistant OCD resulted in improvement in obsessions [Rodriguez CI et al. J Clin Psychiatry 2011]. This led to a randomized, controlled trial [Rodriguez CI et al. Neuropsychopharmacology 2013]. In this double-blind, placebo-controlled, crossover study, 15 patients aged 18 to 55 years with OCD and a YBOCS score of >16 were randomly assigned to receive ketamine or placebo (Phase 1), and then crossed over at least 1 week apart (Phase 2). In the first phase, patients who received ketamine demonstrated a rapid (mid-infusion) reduction in OCD visual analog scale score, which was not observed in patients who received placebo. In addition, 50% of patients in the ketamine arm responded to treatment, whereas 0% in the placebo arm responded.
Dr. Rodriguez concluded that data from these studies suggest that ketamine can rapidly resolve OCD symptoms, which resolution can last for at least 1 week. She suggested that the underlying mechanism of the efficacy of ketamine in OCD is that it repairs a gamma-aminobutyric acid deficit.
Jeffrey Lieberman, MD, Columbia University, New York, New York, USA, presented data on the novel antipsychotic, ITI-007. It has multiple mechanisms of action including 5-HT2A receptor antagonist, dopamine phosphoprotein modulator, glutamatergic phosphoprotein modulator, and serotonin reuptake inhibitor. In addition, ITI-007 is metabolized by ketone reductase to its major metabolite, IC200131, which is an antagonist of 5-HT2A receptor and inhibits the serotonin transporter.
ITI-007 was evaluated in a double-blind, placebo- and active-controlled, Phase 2 trial, in which 335 patients with a current acutely exacerbated episode of schizophrenia were randomly assigned to receive one of four treatments: ITI-007 60 mg, ITI-007 120 mg, risperidone 4 mg (active control), or placebo. The primary outcome was PANSS at Day 28. Patients in the risperidone and ITI-007 60-mg arms demonstrated a significant decrease in total PANSS score compared with placebo over 28 days (p≤0.05). In addition, a subgroup analysis of patients with depression demonstrated that treatment with 60 mg of ITI-007, but not risperidone, decreased total PANSS score compared with placebo. Common adverse events included sedation/somnolence, dry mouth, dizziness, tachycardia, diarrhea, and akathisia; however, the frequency was nonsignificantly different from placebo in the ITI-007 60 mg and risperidone arms. Compared with risperidone, ITI-007 caused lower change from baseline in metabolic parameters such as blood glucose and insulin levels.
Dr. Lieberman concluded that the data suggest that ITI-007 is safe and effective in patients with schizophrenia. In addition, its multitargeted mechanism of action represents a new approach for the treatment of schizophrenia.
Joseph R. Calabrese, MD, Case Western Reserve University School of Medicine, Cleveland, Ohio, USA, discussed new data for pharmacotherapy of bipolar depression. A recent double-blind trial randomly assigned patients with bipolar I depression to receive adjunctive lurasidone or placebo in addition to lithium or valproate for 6 weeks [Loebel A et al. Am J Psychiatry 2014]. The mean change from baseline was significantly better beginning at 3 weeks for patients who received adjunctive lurasidone compared with placebo (p<0.001). Similarly, patients with bipolar depression who received lurasidone monotherapy in a similar trial demonstrated a significantly greater mean change from baseline compared with placebo (p<0.05) [Loebel A et al. Am J Psychiatry 2014]. In both trials, a significant improvement in change from baseline of Montgomery-Asberg Depression Rating Scale (MADRS)-10 compared with placebo was demonstrated for apparent and reported sadness, sleep, lassitude, inability to feel, and pessimistic thoughts (p<0.05). For adjunctive lurasidone treatment, metabolic parameters such as weight, body mass index (BMI), lipids, and blood glucose were similar to placebo, except for triglycerides, which increased from baseline compared with placebo. For lurasidone monotherapy, the higher dose (80 mg to 120 mg), but not the lower dose (20 mg to 60 mg), of lurasidone resulted in an increase in weight, BMI, and total cholesterol compared with placebo.
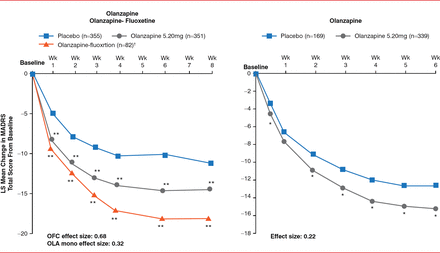
Olanzapine and quetiapine were also evaluated in the treatment of bipolar depression. Previous trials demonstrated that quetiapine treatment in patients with bipolar depression resulted in a significant improvement in MADRS scores compared with placebo over 8 weeks (p<0.05); however, quetiapine was also associated with high rates of sedation and somnolence [Calabrese JR et al. Am J Psychiatry 2005; Thase ME et al. J Clin Psychopharmacol 2006]. Treatment with olanzapine in combination with fluoxetine, as well as olanzapine monotherapy, resulted in a significant improvement in MADRS compared with placebo over 8 weeks (Figure 1) [Tohen M et al. Arch Gen Psychiatry 2003; Br J Psychiatry 2012]. However, olanzapine is associated with increased appetite and weight gain compared with placebo.
Effect of Olanzapine on MADRS in Patients With Bipolar Depression
1 Olanzapine-fluoxetine dosing: 6 and 25 mg; 6 and 50 mg; or 12 and 50 mg;*p<0.05; **p<0.01 vs placebo; LS=least squares; M ADRS=Montgomery-Asberg Depression Scale; mono=monotherapy; OFC=olanzapine-fluoxetine; OLA=olanzapine.
Reproduced from Tohen M et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003;60(11):1079–1088. With permission from the American Medical Association.
In addition to quetiapine and olanzapine, lurasidone and ITI-007 appear to represent an additional option in the treatment of bipolar depression. Although more studies with a larger population are needed, minocycline and ketamine may be effective in pediatric and adult patients, respectively, with OCD.
The editors would like to thank the many members of the 2014 American Psychiatric Association presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®