Summary
Treatment with prolonged-release fampridine results in sustained, clinically meaningful improvement in walking ability and balance in patients with multiple sclerosis (MS). This article presents the results of Exploratory Study to Assess the Effect of Fampridine on Walking Ability and Balance in Patients With Multiple Sclerosis (MOBILE; NCT01597297), an exploratory, phase 2, randomized, double-blinded, placebo-controlled trial.
- Demyelinating Diseases Clinical Trials
- Neurology
- Demyelinating Diseases
- Neurology Clinical Trials
Treatment with prolonged-release (PR) fampridine results in sustained, clinically meaningful improvement in walking ability and balance in patients with multiple sclerosis (MS), said Jan Lycke, MD, University of Gothenburg, Gothenborg, Sweden, who presented the results of Exploratory Study to Assess the Effect of Fampridine on Walking Ability and Balance in Patients With Multiple Sclerosis (MOBILE; ClinicalTrials.gov identifier NCT01597297), an exploratory, phase 2, randomized, double-blinded, placebo-controlled trial.
Among patients with MS, difficulty walking is a commonly reported disability that negatively affects quality of life. PR fampridine (also known as sustained- or modified-release fampridine and dalfampridine extended release) is the only drug currently approved to improve walking in patients with MS. The objective of the MOBILE study was to evaluate the effect of PR fampridine on self-assessed walking ability, dynamic and static balance, and quality of life for patients with MS.
Patients in the MOBILE study had primary or secondary progressive MS, progressive-relapsing MS, or relapsing-remitting MS and Expanded Disability Status Scale scores of 4 to 7. Walking ability was assessed using the 12-item Multiple Sclerosis Walking Scale (MSWS-12) and the Patient Global Impression of Change scale. Mobility and balance (dynamic and static) were assessed using the Timed Up and Go (TUG) test and the Berg Balance Scale. The physical subscale of the 29-item Multiple Sclerosis Impact Scale and the EuroQol 5–Dimension 5-Level instrument were used to evaluate quality of life. Clinically meaningful improvement was defines as ≥8-point mean improvement on the MSWS-12 and 315% mean improvement on the TUG test.
A total of 132 patients from 24 sites were randomized to treatment with PR fampridine (10-mg tablets; n=68) or placebo (n=64) twice daily for 24 weeks. Subjects' mean age was 49.8 years, and their mean Expanded Disability Status Scale score was 5.7. About half (54%) were women. The majority of subjects had either relapsing-remitting MS (33%) or secondary progressive MS (52%).
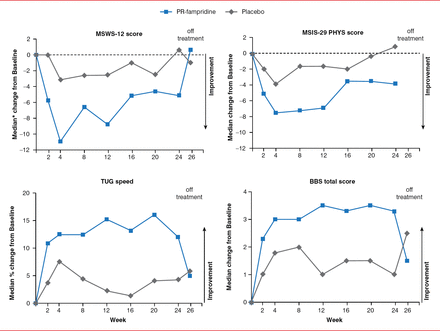
Over the 24-week study, significantly more subjects randomized to PR fampridine experienced clinically meaningful improvements on the MSWS-12 (48.5% vs 28.1%, p=0.015) and TUG speed (47.1% vs 30.2%; p=0.026) versus placebo. PR fampridine treatment also resulted in greater median improvements from baseline on the MSWS-12 (−6.92 vs −2.89), the TUG speed (12.26% vs 3.49%), the Berg Balance Scale (2.93 vs 1.71), and the 29-item Multiple Sclerosis Impact Scale physical subscale (−4.96 vs −2.19) versus placebo. After discontinuation of treatment at Week 24, scores returned to baseline levels by Week 26 (Figure 1).
Median Change From Baseline by Study Visit
BBS=Berg Balance Scale; MSIS-29 PHYS=29-item Multiple Sclerosis Impact Scale-Physical subscale; MSWS-12=12-item Multiple Sclerosos Walking Scale; PR=prolonged release; TUG=Timed Up and Go.
Reproduced with permission from I. Lycke, MD.
*On December 1, 2014, this was changed from Media to Median.
Between-group differences in the results for the Patient Global Impression of Change were reported only at the week 2 visit but were not significant. There was no clear difference between the 2 groups on the EuroQol 5-Dimension 5-Level at any time point.
Safety findings were consistent with the known safety profile of PR fampridine. Nasopharyngitis was the most frequently reported adverse event in the PR fampridine group, while urinary tract infection was the most common adverse event for placebo-treated patients.
This study demonstrated benefits beyond those seen in the phase 3 PR fampridine studies. Potentially, that was related to this trial's longer double-blind, placebo-controlled treatment period and its broader range of objective and patient-reported measures of walking ability.
- © 2014 MD Conference Express®