Summary
This article presents results of the Ranolazine in Atrial Fibrillation Following an Electrical Cardioversion randomized trial [RAFFAELLO; NCT01534962], an international, double-blind, parallel, Phase 2, dose-ranging study testing three oral ranolazine doses. The results demonstrated safety and provided favorable findings with regard to efficacy for patients with atrial fibrillation.
- Cardiology Clinical Trials
- Arrhythmias
- Cardiology Clinical Trials
- Arrhythmias
- Cardiology
Gaetano De Ferrari, MD, Policlinico S Matteo and University of Pavia, Pavia, Italy, presented results of the Ranolazine in Atrial Fibrillation Following an Electrical Cardioversion randomized trial [RAFFAELLO; NCT01534962], an international, double-blind, parallel, Phase 2, dose-ranging study testing three oral ranolazine doses. The results demonstrated safety and provided favorable findings with regard to efficacy for patients with atrial fibrillation (AF).
Although antiarrhythmic medications are widely used in managing patients with AF, with the aim of reducing mortality and hospitalizations, their use has been limited by a combination of toxicity and only modest efficacy. Ranolazine is a relatively new drug approved for the management of chronic angina that blocks late sodium currents, and although it also reduces supraventricular arrhythmias, its use by patients with AF is poorly documented [Zimetbaum P. Circulation 2012].
The RAFFAELLO trial conducted by Prof. De Ferrari and colleagues was the first study to prospectively examine the efficacy and safety of ranolazine in patients with persistent AF. To be included in the study, patients were required to be aged ≥18 years, have persistent AF of 7 days to 6 months in duration, and be suitable for direct-current cardioversion (DCC). Exclusion criteria included congestive heart failure (NYHA Class 3 or 4) and the use of Class 1 or 3 antiarrhythmic agents in the previous 3 days (or 2 weeks and 3 months in the cases of dronedarone and oral amiodarone, respectively).
The primary end point was median time from randomization to first documented AF recurrence. Secondary end points included time to first documented and confirmed AF recurrence and time to first documented AF recurrence in patients who remained in sinus rhythm 2 days after DCC.
During the 16-week study, electrocardiograms (ECGs) were performed daily and in the case of symptoms, using transtelephonic ECG (TT-ECG) devices. ECGs were then transmitted to a central core ECG laboratory for interpretation.
Intention-to-treat (ITT) analysis was performed on 238 study participants, who were randomly assigned 1:1:1:1 to ranolazine (RAN) 375 mg twice daily (BID) (n=65), 500 mg BID (n=60), or 750 mg BID (n=58), or placebo (n=55). The most common ECG interpretation was stable sinus rhythm (79.1%), followed by AF (11.5%), and the remaining 9.3% comprised poor-quality recordings that were unable to be assessed.
Overall safety was favorable, with a similar incidence of all treatment-emergent side effects in the RAN 375 (78.5%), RAN 500 (76.7%), RAN 750 (72.4%), and placebo (74.5%) groups. The incidence of drug-related side effects appeared to be dose dependent (10.8% vs 15.0% vs 22.4% vs 3.6%), was mostly mild, and involved dizziness, fatigue, constipation, and nausea. Severe side effects were rare, comprising pancreatitis (RAN 375; n=1), orthostatic hypotension (RAN 750; n=1), atrial flutter (RAN 750; n=1), and sudden cardiac death (placebo; n=1).
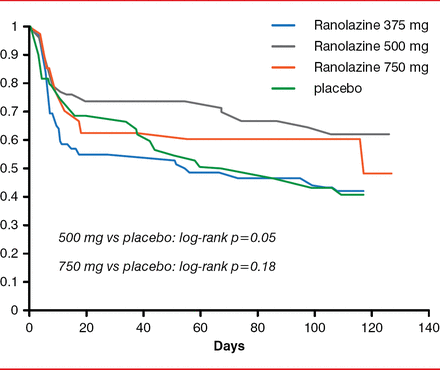
The primary efficacy endpoint of this study was not met, because no single dose of ranolazine significantly prolonged time to first AF recurrence. In a prespecified analysis of time to first AF recurrence, however, excluding patients who relapsed within the first 48 hours, there was a trend toward significance for the two higher doses of ranolazine (Figure 1).
Prespecified Analysis of Time to First Atrial Fibrillation Recurrence
Reproduced with permission from GF De Ferrari, MD.
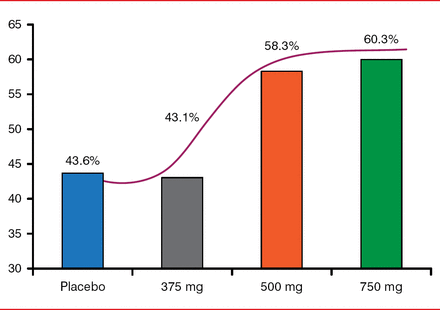
An exploratory analysis of the different dose groups also showed that, whereas the 375-mg dose was ineffective, the two higher doses showed promising trends toward efficacy in freedom from AF (Figure 2).
Freedom From Atrial Fibrillation
Reproduced with permission from GF De Ferrari, MD.
The study did suggest very good safety and tolerability of ranolazine. In addition, combining data from the 500- and 750-mg-dose groups and comparing them with either placebo or the ineffective 375-mg-dose group suggested a 25% to 30% reduction in overall recurrence of AF, concluded Prof. De Ferrari.
These data show favorable findings that support further investigation in the use of ranolazine after cardioversion by patients with atrial fibrillation.
- © 2014 MD Conference Express®