Summary
This article presents results from the Phase 2 Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients With Paroxysmal Atrial Fibrillation trial [HARMONY; NCT01522651]. This randomized, double-blind, placebo-controlled trial demonstrated that a combination of ranolazine and low-dose dronedarone reduced the burden of paroxysmal atrial fibrillation when compared with either drug alone.
- Cardiology Clinical Trials
- Arrhythmias
- Cardiology & Cardiovascular Medicine
- Cardiology Clinical Trials
- Arrhythmias
Peter R. Kowey, MD, Lankenau Medical Center, Wynnewood, Philadelphia, Pennsylvania, USA, presented results from the Phase 2 Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients With Paroxysmal Atrial Fibrillation trial [HARMONY; NCT01522651]. This randomized, double-blind, placebo-controlled trial demonstrated that a combination of ranolazine and low-dose dronedarone reduced the burden of paroxysmal atrial fibrillation (PAF) when compared with either drug alone.
Both ranolazine and dronedarone are drugs that block multiple ion channels. Dronedarone is approved for management of patients with PAF. Ranolazine not only is approved for chronic angina but also has been shown to have antiarrhythmic effects [Mason PK, DiMarco JP. Circ Arrhythm Electrophysiol 2009]. Neither of these drugs, however, has proved very effective for the treatment of PAF when used as monotherapy.
Dr. Kowey and colleagues conducted a study to determine if the combination of ranolazine and low-dose dronedarone is superior to individual drug therapy in reducing the burden of atrial fibrillation (AF) in patients with PAF.
To be included in the trial, patients were required to be aged ≥18 years, with a history of PAF documented within the prior 12 months, and a dual-chamber programmable pacemaker with AF detection capabilities (implanted at least 3 months prior to screening).
Exclusion criteria included persistent or permanent AF, a history of atrial flutter or atrial tachycardia without successful ablation, other acutely reversible causes of AF, a prior heart transplant, or a history of stroke 3 months prior to screening.
In total, 134 patients were enrolled and randomly assigned to five groups: placebo (n=26), ranolazine 750 mg twice daily (BID) (n=26), dronedarone 225 mg BID (n=26), ranolazine 750 mg and dronedarone 225 mg BID (n=27), or ranolazine 750 mg and dronedarone 150 mg BID (n=26).
The primary end points were the relative and absolute changes from baseline in AF burden (AFB) at 12 weeks. Secondary end points included the change in AFB at each study visit, the percentage of patients with a 50% reduction of AFB, and the safety and tolerability of dronedarone and ranolazine when used as monotherapy and/or in combination.
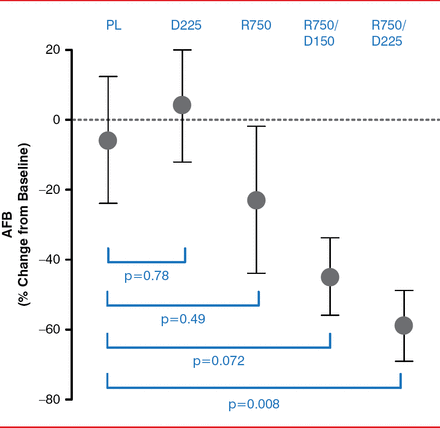
Compared with placebo, the patient group treated with ranolazine 750 mg and dronedarone 225 mg BID had a statistically significant reduction in AFB from baseline to 12 weeks (p=0.008; Figure 1). The reduction in AFB for those in the ranolazine group and in the dronedarone 150 mg BID and ranolazine 750 mg daily group was not statistically significant (p=0.072 and p=0.49, respectively). In addition, the patient group treated with dronedarone 225 mg BID showed no difference in percentage change in AFB when compared to placebo (p=0.78).
Percentage Change in AFB From Baseline to 12 Weeks
AFB=atrial fibrillation burden; D225=dronedarone 225 mg BID; R750=ranolazine 750 mg BID; R750/D150=ranolazine 750 mg and dronedarone 150 mg BID; R750/D225=ranolazine 750 mg and dronedarone 225 mg; PL=placebo.
Reproduced with permission from PR Kowey, MD.
From baseline to 12 weeks, a ≥70% reduction of AFB occurred in 45% and 27% of patients in the ranolazine 750 mg-dronedarone 225 mg BID and ranolazine 750 mg- dronedarone 150 mg BID groups, respectively, compared to an 11% reduction in the placebo group. Neither ranolazine nor dronedarone 225 mg BID alone reduced AFB when compared with placebo (only 17% and 9%, respectively).
There were very few serious adverse events (AEs) reported in the treatment groups, and there was no dose relationship with respect to either serious AEs or those leading to treatment discontinuation. Dizziness and constipation were some of the most frequent serious AEs reported.
In summary, the HARMONY trial showed that ranolazine and dronedarone lowered AFB as compared to placebo or either agent when used as monotherapy. In addition, there was a dose-response relationship seen with the effects of dronedarone when used in combination with ranolazine.
Dr. Kowey concluded by noting that plans are now underway to further study these agents with 2 large Phase 3 trials. One trial plans to study the effects of dronedarone and ranolazine on the time to recurrent atrial fibrillation. The other trial will study the effects of dronedarone and ranolazine on cardiovascular death or hospitalization.
- © 2014 MD Conference Express®