Summary
This article presents results of the prospective, single-center Smartphone Pediatric Electrocardiogram trial [SPEAR] demonstrating that smartphone-enabled electrocardiogram (ECG) devices can produce accurate tracings of diagnostic and therapeutic quality in children in the remote setting.
- Arrhythmias
- Cardiology Clinical Trials
- Imaging Modalities
- Cardiac Imaging Techniques
- Cardiology & Cardiovascular Medicine
Hoang H. Nguyen, MD, Washington University School of Medicine, St. Louis, Missouri, USA, presented results of the prospective, single-center Smartphone Pediatric Electrocardiogram trial [SPEAR] demonstrating that smartphone-enabled electrocardiogram (ECG) devices can produce accurate tracings of diagnostic and therapeutic quality in children in the remote setting.
Smartphone-enabled ECG recorders have the ability to enhance physician reach and patient care by facilitating ECG assessment in patients in remote areas. One such example is the AliveCor 1-lead ECG device, which consists of 2 exposed electrodes on the back of a smartphone case. This device generates an ECG when a finger of each hand is placed on each of the electrodes, and the electrical signal is processed and transmitted to the phone's AliveCor application.
Dr. Nguyen and colleagues are conducting a prospective trial throughout a 1-year period to investigate the usefulness of ECG tracings generated by the AliveCor device in pediatric patients, and to evaluate user satisfaction.
To be included in the trial, patients were required to be aged ≤21 years, have documented paroxysmal arrhythmia, and own an iPhone 4 or 5. Users were instructed to email ECG tracings of concern directly from the application for review by pediatric cardiac electrophysiologists. Following interpretation, patients were contacted with results and further care instructions. They were also required to complete online surveys regarding their experience and satisfaction with the device and cardiac care team.
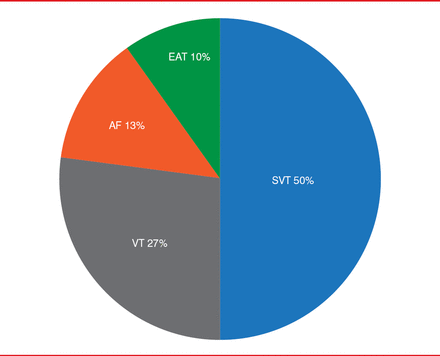
In total, 30 patients (aged 2 months to 18 years; median age 12.5 years) were enrolled in the study. To date, 144 ECG tracings have been received from 20 patients, and the highest number of tracings received from a single patient during a 1-month period was 15. Users deemed ECGs as concerning 45% of the time. Signal quality allowed unequivocal rhythm diagnosis in 141 of 144 (98%) tracings; motion artifact prevented evaluation of the remaining three tracings. The most frequent diagnosis was supraventricular tachycardia (n=15; 50%), followed by ventricular tachycardia (n=8; 27%), atrial fibrillation (n=4; 13%), and ectopic atrial tachycardia (n=3; 10%; Figure 1).
Rhythm Diagnosis From Smartphone ECG Tracings
AF=atrial fibrillation; EAT=ectopic atrial tachycardia; SVT=supraventricular tachycardia; VT=ventricular tachycardia.
Reproduced with permission from HH Nguyen, MD.
Forty-four surveys have also been received to date, 68% of which are from parents. The results of the user survey thus far have been positive, with 98% of users indicating that the device is very easy to use and 93% indicating the ease at which it transmits tracings. Of users, 98% expressed a high level of comfort in using the device for arrhythmia management, and 99% indicated continued interest in using it after the study ends.
The parents of younger patients also provided feedback noting that the device can record their child's heart rhythm for prompt diagnosis without the need to visit the emergency room (ER) or pediatrician's office. They did, however, indicate the difficulty of device placement on small children. In addition, some noted that because the device was on the parental phone, it was not with the child at all times.
These preliminary data demonstrate that smartphone-enabled ECG devices can produce diagnostic tracings in children, with high use and user satisfaction. Such devices can help pediatric electrophysiologists better manage chronic arrhythmia by optimizing pediatric outpatient care, limiting ER use, and thereby reducing health care costs, concluded Dr. Nguyen.
- © 2014 MD Conference Express®