Summary
The microalbuminuria model of nephropathy in patients with type 1 diabetes mellitus (T1DM) may no longer be valid and may be replaced by the model of early progressive renal decline (EPRD), according to new data that support the latter. This article discusses EPRD as the new paradigm in nephropathy in patients with T1DM.
- Diabetes Mellitus Diabetes & Kidney Disease
- Renal Disease
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes & Kidney Disease
- Renal Disease
The microalbuminuria (MA) model of nephropathy in patients with type 1 diabetes mellitus (T1DM) may no longer be valid and may be replaced by the model of early progressive renal decline (EPRD), according to new data that support the latter. Andrzej S. Krolewski, MD, PhD, Joslin Diabetes Center, Boston, Massachusetts, USA, discussed EPRD as the new paradigm in nephropathy in patients with T1DM.
MA was the primary focus of the model of nephropathy in patients with T1DM in the 1990s, in which MA was considered to be the initial sign of kidney disease and both MA and proteinuria were considered a disease process. However, new insights have come from the first Joslin Kidney Study, conducted between 1991 and 2006, and the second Joslin Kidney Study, conducted between 2003 and 2014. The dataset for these studies comes from the longitudinal, observational Joslin Study of the Natural History of Microalbuminuria [Perkins BA. N Engl J Med 2003], which included about 3000 patients with T1DM who contributed DNA, RNA, plasma, serum, and urine to a Biobank for ongoing research.
The first Joslin Kidney Study, in which 386 patients were followed for 6 and 12 years, revealed that MA was a functional abnormality that can regress; only a small number of patients progressed to proteinuria, thus contradicting the accepted MA model.
Analyzing a subset of the MA cohort, investigators found that in 79 patients with new-onset MA, 55 had stable renal function for up to 14 years and 24 (30%) experienced progressive renal decline, as measured by a decrease in estimated glomerular filtration rate (eGFR) [Merchant ML et al. J Am Soc Nephrol 2009]. According to the 1990s model of MA, the majority of these patients should have progressed to proteinuria before losing renal function. Dr. Krolewski stated that EPRD precedes the development of proteinuria and even MA, as demonstrated in the recent study.
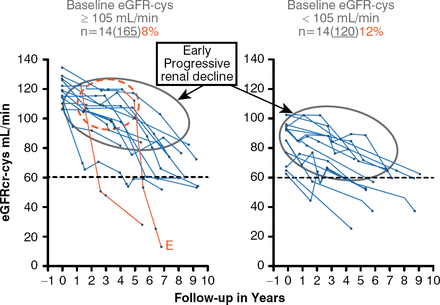
The second Joslin Kidney Study revealed that 10% of 280 patients with T1DM and normal albuminuria (NA) experienced EPRD, as defined by an eGFR loss of greater than 3.3% mL/minute per year over 6 to 10 years of follow-up (Figure 1) [Krolewski AS et al. Diabetes Care 2014]. Interestingly, this decline already was underway in patients with baseline eGFR below 105 mL/minute (median), whereas patients with baseline eGFR above median experienced renal decline after several years of stable renal function. The numbers of patients were small, but in the subjects with baseline eGFR above median, the investigators observed the onset of EPRD (the red circle in Figure 1) after which the process progressively led to renal failure, but at a rate that was extremely variable among individuals. One patient reached end-stage renal disease within 2 years, whereas others may reach this point in 30 years if they continue progressive renal decline at the same rate.
Early Progressive Renal Decline in Patients With Type 1 Diabetes
eGFRcr-cys = estimated glomerular filtration rate by creatinine and cystatin C.
Reproduced from Krolewski AS et al. Early Progressive Renal Decline Precedes the Onset of Microalbuminuria and Its Progression to Macroalbuminuria. Diabetes Care 2014;7:226–234. Copyright © 2014 American Diabetes Association. All rights reserved.
Dr. Krolewski concluded that EPRD begins in patients with normal renal function (eGFR > 105 mL/minute) as the primary manifestation of nephropathy regardless of MA status. Once patients present with early renal decline, they will progress to end-stage renal disease (ESRD) within 2 to 30 years, and the trajectories of this progression can be approximated as eGFR slopes. The lack of animal models of EPRD requires that the study of its determinants and mechanisms be conducted in humans.
At the Joslin Clinic, Dr. Krolewski and his colleagues are using materials from the Biobank collected from patients in the Joslin Kidney Studies to identify markers or predictors for EPRD. In a subset of 462 patients from the second Joslin Kidney Study followed 6 to 10 years, levels of plasma kidney injury molecule (KIM)-1, a marker of proximal tubule damage, were associated with a linear increase in the risk of developing EPRD in patients who initially had NA or low to high MA [Nowak et al. 2014. Submitted for publication]. Other independent predictors of risk of EPRD are urinary monocyte chemoattractant protein (MCP)-1 and epidermal growth factor (EGF). Dr. Krolewski noted that urinary markers are probably a consequence of kidney injury rather than a causal factor, and tubular damage and inflammation likely are important intermediate factors in EPRD.
To determine potential causal factors involved in EPRD, genome-wide association studies (GWAS) have been conducted in Dr. Krolewski's laboratory. A GWAS conducted in 623 patients with T1DM identified 13 potential single nucleotide polymorphisms associated with eGFR slopes [Skupien J et al. Unpublished data]. These findings will be examined further in 4 cohorts totaling 2010 patients in whom eGFR slopes were estimated (800 progressed to ESRD) to find genes that determine the rate of eGFR decline and time of onset of ESRD in patients with T1DM and proteinuria.
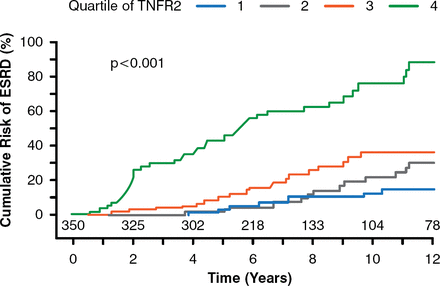
New systemic markers of EPRD are being elucidated using metabolomics, global proteomics, and targeted proteomics by members of Dr. Krolewski's laboratory. A targeted proteomics study found that circulating tumor necrosis factor receptor (TNFR) types 1 and 2 were predictors of ESRD in patients with type 2 diabetes mellitus (T2DM) [Niewczas MA et al. J Am Soc Nephrol 2012]. In another study, involving 349 patients with T1DM and proteinuria, patients with the highest quartile of TNFR 2 serum concentration had the highest rate of eGFR loss and the highest risk of developing ESRD (p< .001; Figure 2) [Skupien J et al. Diabetes Care 2014]. The mechanism of the correlation between TNFR 1 and 2 and risk of ESRD is unknown, but lowering serum TNFR levels may be a therapeutic target to postpone the onset of ESRD in patients with DM, stated Dr. Krolewski.
Tumor Necrosis Factor Receptor 2 as a Predictive Marker of End-Stage Renal Disease
ESRD = end-stage renal disease; TNFR2 = tumor necrosis factor receptor 2.
Reproduced from Skupien J et al. Synergism Between Circulating Tumor Necrosis Factor Receptor 2 and HbA1c in Determining Renal Decline During 5–18 Years of Follow-up in Patients With Type 1 Diabetes and Proteinuria. Diabetes Care 2014; doi:10.2337/dc13-1983. Copyright © 2014 American Diabetes Association. All rights reserved.
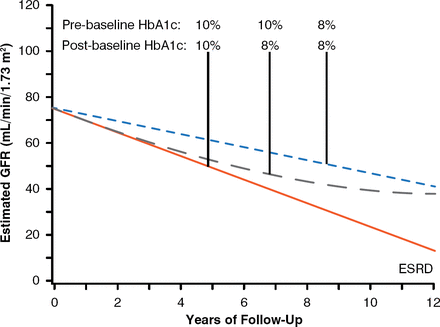
To examine whether long-term improvement of glycemic control affects the trajectories of eGFR in patients with EPRD, a cohort of 349 patients with T1DM and proteinuria were followed for 7 to 15 years to estimate eGFR slopes and ascertain onsets of ESRD. Almost half of them had EPRD. About 30% of patients had improved glycemic control once they developed proteinuria. To determine the effect of improved glycemic control during follow-up, average HbA1c during the 5 years before study baseline (preproteinuria or prebaseline) was compared with HbA1c (postbaseline) averaged during the first half of follow-up (median 5.1-year interval). Differences between these averages were examined for association with renal outcomes. Median preproteinuria HbA1c was 9.3% and decreased to 8.7% postbaseline. Cumulative risk of ESRD after 15 years was significantly lower for those whose HbA1c decreased than for those whose HbA1c increased or remained poor (29% vs 42%, p< .001). The difference in ESRD risk between these groups was not visible at 5 years of follow-up but became visible at 10 and 15 years of follow-up [Skupien J et al. J Am Soc Nephrol 2014]. Patients who had sustained improvements in HbA1c demonstrated a reduced rate of eGFR decline over 12 years that was independent of reno-protective drugs. The effects of improvements in HbA1c from 10% to 8% are shown in Figure 3. In patients with T1DM and proteinuria, sustained improvement in glycemic control delayed ESRD onset by 6 to 10 years. In conclusion, this observational study suggests that long-term sustained improvement in HbA1c decelerates eGFR loss and delays the onset of ESRD of patients with T1DM and proteinuria.
Effect of Glycemic Control on Risk of Developing End-Stage Renal Disease
ESRD = end-stage renal disease; GFR = glomerular filtration rate.
Reproduced from Skupien J et al. Improved Glycemic Control and Risk of ESRD in Patients With Type 1 Diabetes and Proteinuria. JASN 2014; doi:10.1681/ASN.2013091002. Reproduced with permission from the American Society of Nephrology.
Dr. Krolewski concluded that the MA model of nephropathy in patients with diabetes is now outdated and should be replaced with the EPRD model. This model provides a new context for studying the cause of diabetic nephropathy, searching for new predictors, and developing new interventions. Meanwhile, patients with EPRD can be identified early by measuring serum TNFR 1 and other potential markers and should be managed by maintaining their HbA1c levels below 8.0%.
The editors would like to thank the many members of the 2014 American Diabetes Association presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®